Best Practices for Mobile Dental Operations
It might seem counterintuitive, but dental practices afford a certain level of luxury. Those sitting in the dentist chair may not equate a dental practice with luxury-especially when the handpiece fires up-but, certainly, it is a simpler, easier to manage environment for practice staff, especially when compared to mobile dental operations.
Those mobile operations are, of course, just the sort of thing that hard-to-reach patients need. Those who may not be able to come to a permanent facility are well-served by these mobile operations. But those facilities might require extra care, for some functions. Take, for example, infection control. Mobile operations are able to achieve the same level of infection control surely, but it is not always achieved in the same way.
Get your motor running
“Mobile dental units have come a long way,” Karen Daw, “The OSHA Lady”, speaker and consultant, observes. “Modern coaches are now outfitted with water filtration systems, CO2/smoke detectors, fire extinguishers, first aid kits, etc. And, for the most part, infection control considerations for mobile dental units are the same as for brick-and-mortar operations. In other words, the site would still follow CDC and OSHA. There will be weekly biological monitoring of sterilizers, training for all team members upon hire, annually and when there have been changes in processes, proper use of PPE, etc.”
Administrative concerns are an unexpected, but real, issue for mobile operations.
“One area that can be problematic is what to do in the event of an exposure,” Daw says. “Mobile clinics are often traveling, so the post-exposure protocol may not have a specific location where the evaluation will be performed. Sometimes the nearest facility is several hours away, depending on where the mobile unit is scheduled for the day. This might make immediate evaluation difficult. It would be prudent to know in advance the location of the closest medical facility for post-exposure management (and other medical emergencies), how patient testing will be conducted, and whether post-exposure prophylaxis medications are available onsite especially if in a remote area. If at a school location, it should be discussed how to obtain immediate consent from the parent to test a child at a school location.”
Space
Practically speaking, the space limitations afforded by mobile dental operations can test an operation’s effective infection control efforts.
“Another challenge may be PPE (personal protective equipment) ,” Daw says. “Because of limited storage, a routine check must be performed to ensure that there are enough gloves, masks and disposable gowns to last through the day. This is in addition to instruments and other supplies needed. If scrub jackets or lab coats are used, then there must be consideration as to how they will be laundered. According to OSHA, it is the employer’s responsibility to maintain PPE at no cost to the employee.”
Those space limitations might also extend to sterilization equipment.
“And what if there is no sterilizer on board?” Daw says. “How will instruments be maintained, contained and transported for cleaning and sterilization? Who is responsible for ensuring that weekly biological testing is being performed and verifying passing tests? What if the sterilizer fails a cycle while away from home base? When I consult with group practices, some with satellite locations and without on-site sterilization, we create an OP that addresses what is acceptable, with regards to how this will be managed. Because stories related to improper instrument reprocessing make it into the news so often, this is one area where we ensure there is no doubt what is expected.”
Waterline safety concerns have been in the news for permanent facilities, however, those issues are also present for mobile facilities.
Continued reading on the next page
“Water safety is a trending topic in dental infection control and safety,” Daw says. “I once consulted with a dental college’s mobile unit, because their water lines were failing. Turns out, the reservoir tank was not being emptied routinely and the dental unit water lines were not being treated with any product designed to keep bacterial levels within a safe range. The team thought that because they were mobile and refilling water from ‘clean’ sources, and because they were small and compact, that the water was fine. However, the opposite is true. In fact, the design of the mobile unit actually made it an ideal breeding ground for bacteria.”
Space concerns also extend to the area in which staff operates.
“Some might consider the size of the mobile unit more convenient with regards to disinfecting surfaces (as in, there are fewer surfaces to contend with) ,” Daw says. “However, whereas brick-and-mortar locations typically have contracted services to clean clinical and housekeeping areas and surfaces, that typically is not the case for mobile dental units. This means that the team must ensure that all surfaces are cleaned, and, if applicable, disinfected routinely. This is part of the site’s Exposure Control Plan and details how and how often surfaces are to be addressed.”
Guidance
Mobile operations function at varying levels of patient contact, but they all have at least one thing in common: They are bound by the CDC’s 2003 publication, Guidelines for Infection Control in Dental Health-Care Settings. On its website, the Organization for Safety, Asepsis and Prevention (OSAP), offers guidance for mobile dental operations to ensure good infection prevention outcomes.
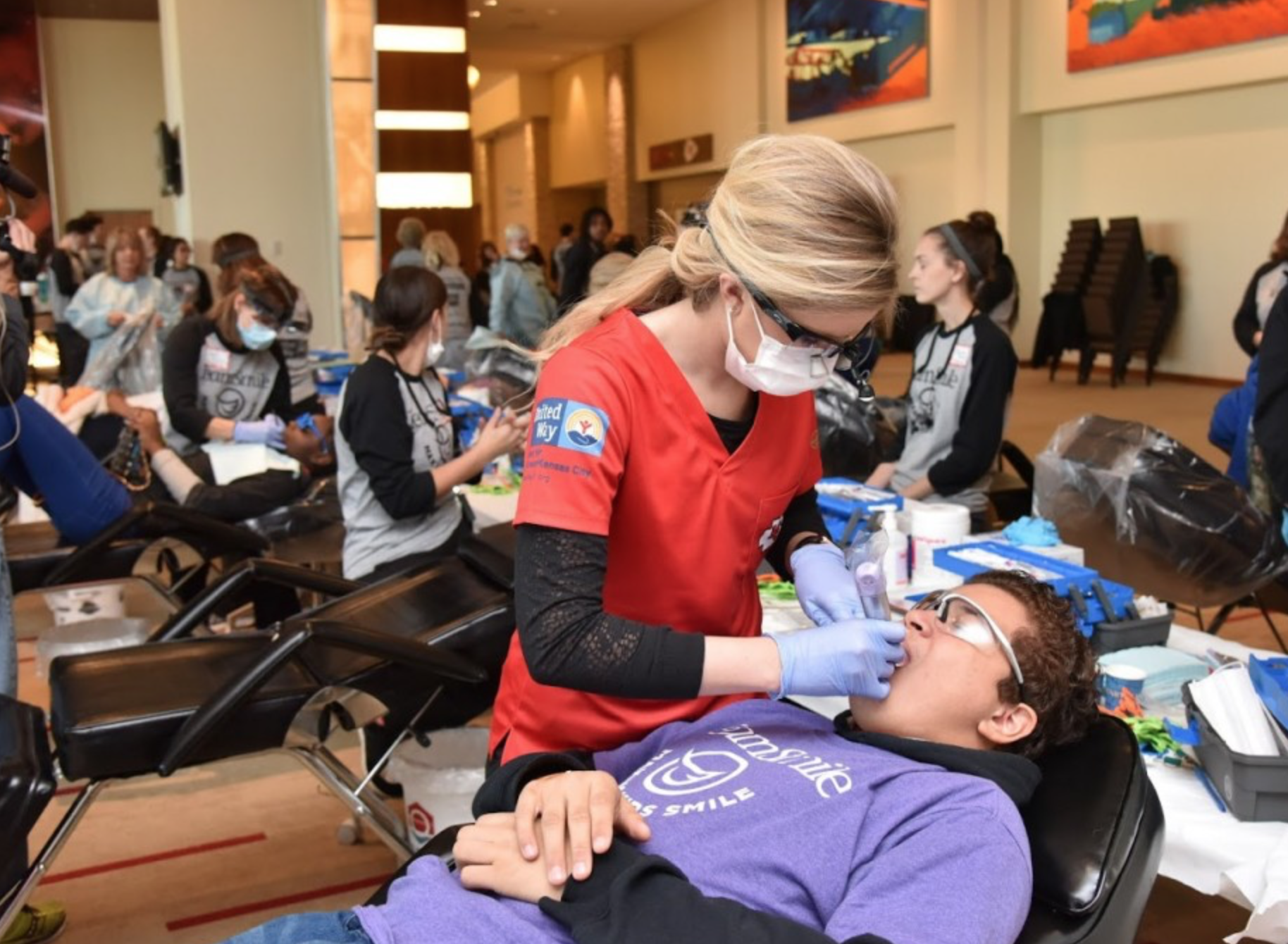
TeamSmile hosts dental care outreach programs around the country and has regular infection control protocols in place.
“Although the 2003 recommendations are applicable to all settings in which dental treatment is provided, the recommendations focus mainly on dental settings that use traditional, fixed equipment (e.g., private practice dental settings),” writes OSAP. “In contrast, a variety of non-traditional dental settings, such as school-based dental programs, use portable dental equipment. These programs often operate in challenging settings. For example, hallways, gymnasiums, or other high-traffic locations may be the only space available for dental screenings or treatment. Additional guidance may be useful in these unique situations, where space and resources needed to comply with recommended infection control practices may be limited (e.g., absence of sinks) or other challenges exist.”
OSAP recommends first performing a site analysis, which includes evaluating such aspects as:
- Adequate space for sharps disposal
- Adequate space for safe handling of Medical waste (regulated and non-regulated)
- Adequate space for Instrument cleaning and processing or secured holding area
Their complete site analysis checklist can be found here.
OSAP offers another checklist that can help mobile dental operations ensure effective infection control. The checklist is helpful in analyzing whether or not mobile facilities are prepared with proper infection control protocols. It starts by analyzing levels of anticipated contact between the dental health care professional (DHCP) and the patient’s mucous membranes, blood or saliva visibly contaminated with blood to determine the suggested elements for the infection control program. The checklist is designed to provide information for three levels of programs:
i. Anticipated contact with the patient’s mucous membranes, blood or saliva visibly contaminated with blood.
ii. Anticipated contact with the patient’s mucous membranes but not with blood or saliva visibly contaminated with blood.
iii. No anticipated contact with the patient’s mucous membranes, blood, or saliva visibly contaminated with blood.
Checklist items include:
- Is there a written procedures manual for post-exposure management?
- Are sharps containers safely located as close as possible to the user?
- Is there a list of which housekeeping surfaces will need to be cleaned and disinfected and how often?
The complete checklist can be found here.
One size doesn’t fit all
Mobile dental operations are not all exactly the same. Because each practice services unique patients in different settings, their needs will be different.
For instance, consider TeamSmile. The organization visits stadiums across the country, setting up dental facilities for children who need dental care.
“We’re not your typical mobile unit in terms of, ‘We’re bringing a mobile unit and somebody's coming out of your truck,’” TeamSmile Executive Director John McCarthy says. “We carry around $600,000 to $700,000 of dental equipment and set them up in the stadiums.”
TeamSmile sets up 16 hygiene stations and 12 dental operatories at each function.
“We have everything from check-in and check-out, x-rays, sterilization-it’s quite a thing,” McCarthy says.
While they bring their equipment from arena to arena, packing-in and packing-out their equipment, they operate just like a brick-and-mortar facility, including their infection prevention efforts.
“Our sterilization area is set up exactly as you would find in a dental office,” Kellie Reneau-Jardon, TeamSmile’s Program Director says. “We run the same type of sterilization machines. We go through the same process-dirty instruments come in, they’re cleaned, they go through an ultrasonic machine, they are rinsed, and they are bagged. In our world, there is no abbreviating it, because sterilization is a very, very important protocol.
“We always spore test prior to program day,” she continues. “There are no changes at all in our sterilization area.”
Mobile dental facilities certainly pose their own challenges, in terms of infection control-whether it be managing space, cleaning surfaces, or maintaining safe water lines. But, no matter the size or the operational parameters of the unit, infection prevention rules remain the same for those in permanent structures as they are for those on wheels.
