More than 50 million individuals in the United States and 1 billion individuals globally experience some form of sleep disorder and obstructive sleep apnea (OSA), with an estimated 80% of cases undiagnosed.1,2 Mild to moderate OSA is often the result of underdeveloped upper and lower jaws, which can obstruct the airway. Symptoms include mouth breathing, snoring, daytime drowsiness, depression, and chronic pain. Left untreated, OSA can lead to a wide variety of health risks.
“When people stop breathing at night, their body doesn’t get the deep sleep that it needs,” says Tammarie Heit, DDS, International College of Craniomandibular Orthopedics member and general and craniofacial dentist at Avalon Dentistry who sits on the Vivos Therapeutics Clinical Advisory Board. “This can result in myriad illnesses, including high blood pressure, heart failure, stroke, coronary artery disease, and other chronic conditions that can affect quality and length of life.”
Traditional treatments for sleep apnea, including continuous positive airway pressure, a snoring appliance, and surgical implants, are directed at reducing symptoms and require lifelong intervention.
The Vivos System from Vivos Therapeutics, Inc, is an advanced multidisciplinary therapeutic protocol for treating mild to moderate OSA, snoring, and sleep-disordered breathing (SDB) in adults. Offering an all-natural, nonsurgical, and pain-free approach, the system combines the use of oral appliance technology prescribed by specially trained independent dentists with cooperation from their medical colleagues.
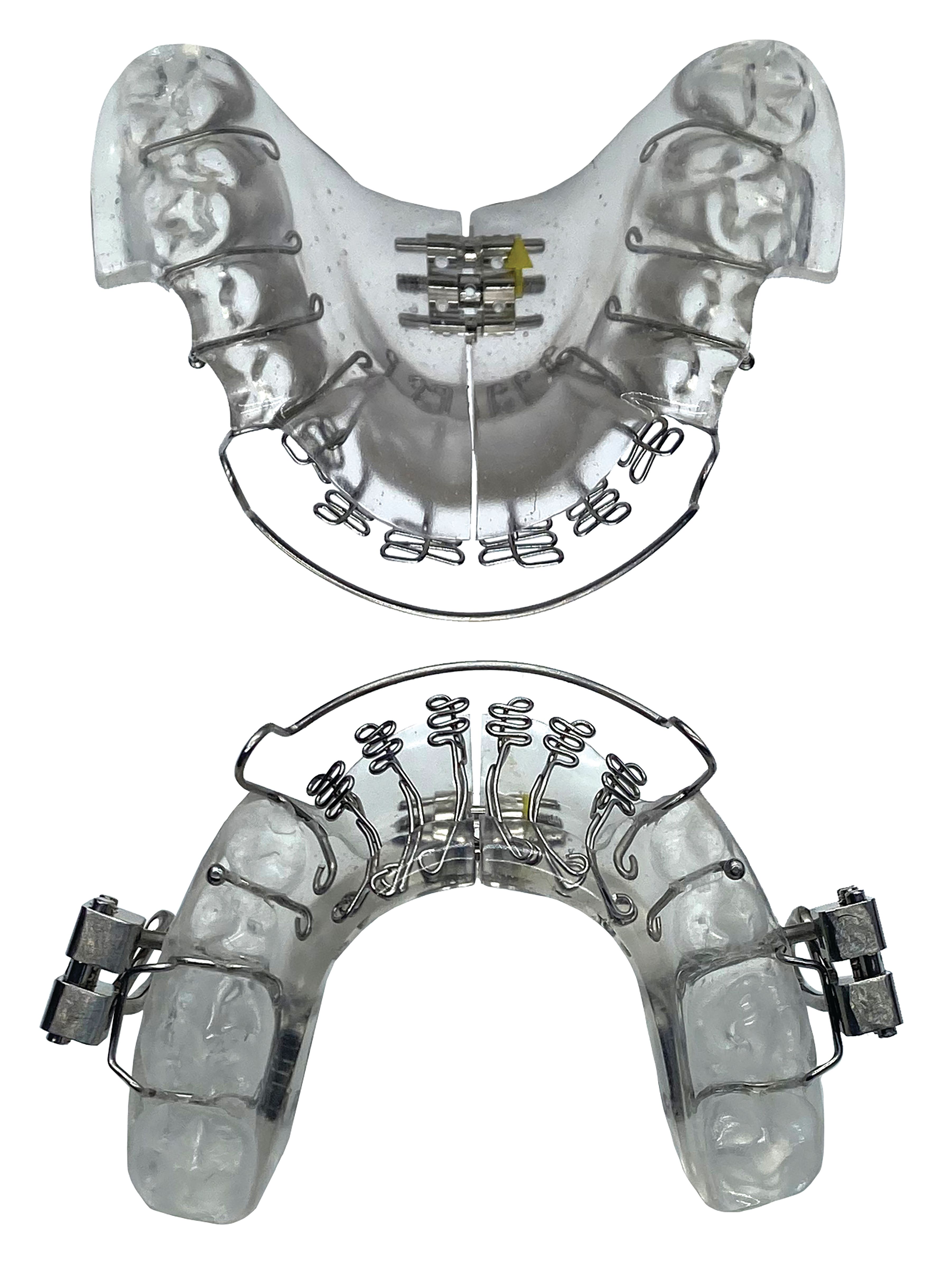
Mandibular Repositioning-Nighttime Appliance (mRNA)
The Vivos System includes the patented mRNA appliance. The proprietary and virtually painless system enhances and increases the upper airway and offers adult patients what Vivos believes to be an effective treatment alternative for mild-to-moderate OSA, snoring and SDB symptoms.
Vivos Therapeutics, Inc
866-908-4867
vivoslife.com
Sleep apnea is a condition that must be diagnosed by a medical professional. “If I suspect that my patient’s airway is collapsing at night, I will screen the patient, including a history and general exam of their mouths and dentition,” says Dr Heit. “If my dental diagnosis is possible sleep apnea, I may also order a sleep study to make it more efficient for my medical colleagues in starting treatment.”
The Vivos System typically begins with a thorough diagnosis of the patient’s condition by a qualified medical doctor working in close collaboration with a specially trained independent dentist. Vivos trains doctors to perform a comprehensive, in-home sleep wellness assessment, including biometric analysis using VivoScore HSAT, which is a single-sensor ring recorder. Precise measurements of the size and volume of the patient’s upper jaw, or maxilla, and their nasal airway volume are obtained. The doctor determines a therapeutic approach and treatment plan to correct craniofacial deficiencies and prescribes a custom oral appliance using a sophisticated algorithm. The system’s appliances include the mRNA appliance®, an FDA-cleared Class II medical device for the treatment of snoring, mild to moderate OSA, and SDB; and the DNA appliance®, an FDA-registered Class I device for palatal expansion.
The patient is instructed to wear the device for 12 to 24 months. During treatment, periodic adjustments to the appliance are made by the dentist.
“The most successful patient wears the device 12 to 16 hours a day in the evening and while sleeping, when growth hormones are higher so the device can change the relationship inside the mouth,” Dr Heit says. “Throughout the night, the device holds the bottom jaw forward, opening up the airway and allowing oxygen in so the body can get the deep sleep it needs.”
Signs that the device is working in expanding the jaw and airway include visible gaps forming between the teeth and more ease of flossing. The patient may also notice fewer headaches, less snoring, better sleep, and an improved overall feeling of well-being.
“As dentists, we can be part of the breathing wellness movement by collaborating with our medical colleagues to get OSA under control,” she concludes.
References
2. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2018;5(2):136-143. doi:10.513/pats.200709-155MG