We may be headed toward a new mouthrinse protocol
Deep Dive: We may be headed toward a new mouthrinse protocol. With the renewed interest in pre-rinsing for dental procedures, chlorine dioxide deserves a closer look.
Information provided by OraCare.
As dental offices across the country look at the steps to take to reopen after the COVID-19 pandemic, having patients pre-rinse has become a must. Pre-rinsing has been shown in many study findings to reduce microbes in the mouth before they become part of dental aerosols. A pre-rinse must kill not only bacteria, but also viruses and fungi. One of the few compounds that can safely accomplish this is activated chlorine dioxide, available from OraCare.
Activated Chlorine Dioxide
Though newer to dentistry, activated chlorine dioxide has been used for disinfection in other industries for nearly 200 years. An important characteristic of this chemical compound is its versatility as a disinfectant, suitable for textile, medicinal, wastewater treatment, public health, food safety, personal hygiene, and household uses. It has even been used against anthrax because it is effective against spore-forming bacteria.1
Chlorine dioxide is a gas and a strong oxidizer. “It effectively kills pathogenic microorganisms such as fungi, bacteria, and viruses. It also prevents and removes biofilm,” according to the website of Lenntech, a water treatment solutions company in The Netherlands. The gas can be potent enough to sterilize medical instruments but mild enough to be used intraorally to fight germs. Chlorine dioxide can be activated in small or large quantities and at varying strengths.
A key point is that “high oxidation potential” means that a chemical reacts with more substances as the power increases. The oxidation power of chlorine dioxide is high, but low enough to prevent it from reacting with cells in a detrimental way.3 As an oxidizer, chlorine dioxide is very selective, so it has a high therapeutic index. In contrast, ozone and peroxide are less discriminating in what they break down.
Another quality of chlorine dioxide is that less is needed to disinfect a given volume of water or saliva than required with some higher-power oxidants (ozone and peroxide). A molecule such as ozone will react with many other contents of saliva and be used up before it has a chance to react with the pathogen. This is why in many instances, including water treatment, chlorine dioxide is preferred over higher-power ozone.
The chlorine dioxide reacts with the volatile sulfur compounds and bacteria but ignores other organic compounds that don’t require treatment. So, even though a product with a high potential strength theoretically would kill at lower concentrations, in reality, it requires a higher concentration to do the job compared with chlorine dioxide.
Its high oxidation abilities also make chlorine dioxide an ideal choice for dental use. Chlorine dioxide has an oxidation capacity of 5e, which means it can accept five electrons from the molecule it is oxidizing. Hydrogen peroxide and ozone can accept just two; thus, chlorine dioxide’s oxidation capacity is 2.5 times higher. Simply stated, it strips off more than twice as many electrons from a pathogen. This occurs in a two-step process: First, the reaction causes chlorine dioxide to be reduced to sodium chlorite. Then the sodium chlorite is reduced to sodium chloride, or ordinary table salt and water, which are harmless. The chlorine molecule remains in the substance until the end; that’s why chlorine dioxide does not produce harmful chlorinated substances such as trihalomethanes.
Chlorine dioxide is sometimes confused with chlorine bleach, but they differ not only in structure but also in behavior. As an oxidizer, chlorine dioxide is very selective compared with traditional chlorine bleach. When bacteria are eliminated using chlorine dioxide, the cell wall is penetrated.
“Bacterial cells react with chlorine dioxide, causing several cellular processes to be interrupted. Chlorine dioxide reacts directly with amino acids and the RNA in the cell. It is not clear whether chlorine dioxide attacks the cell structure or the acids inside the cell, but the production of proteins is prevented. Chlorine dioxide affects the cell membrane by changing membrane proteins and fats and by prevention of inhalation,” according to Lenntech.
At minimum, chlorine dioxide is as effective a bactericide as chlorine, but in many cases it is superior. Specifically, it excels in its role as a virucide.
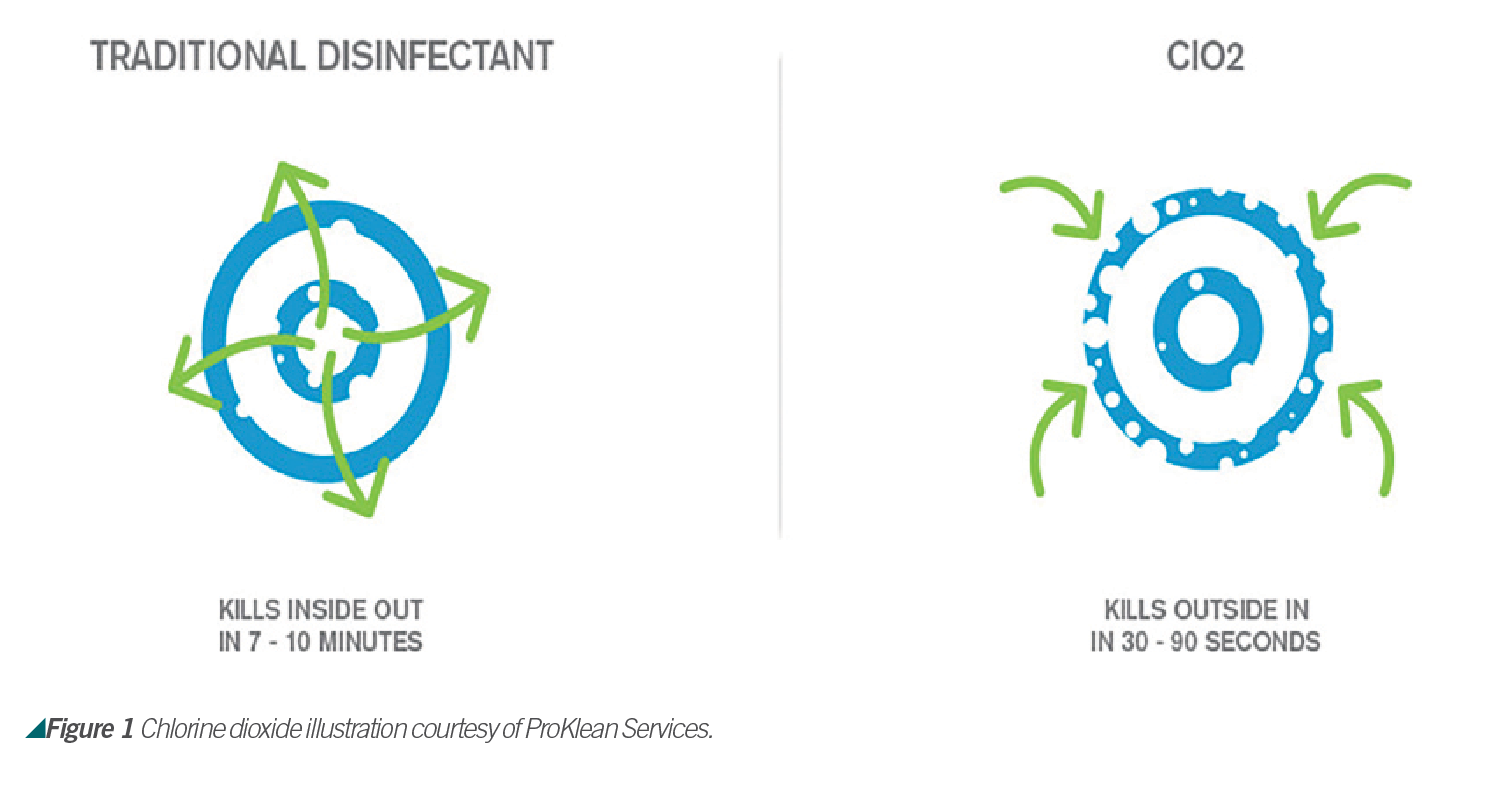
Chlorine Dioxide and Viruses
To kill viruses, chlorine dioxide prevents protein formation by reacting with peptone, a water-soluble substance that originates from hydrolysis of proteins to amino acids. According to Lenntech, bacterial cells react with chlorine dioxide, interrupting several cellular processes. Chlorine dioxide reacts directly with amino acids and the RNA in the cell. It is not clear whether chlorine dioxide attacks the cell structure or the acids inside the cell, but the production of proteins is prevented. Chlorine dioxide affects the cell membrane by changing membrane proteins and fats and by prevention of inhalation (see Figure 1).
Chlorine dioxide’s radical nature makes it an excellent agent in a large pH range. The permeability of living cell walls to gaseous chlorine dioxide radicals seems to increase, allowing easier access to vital molecules.2
The U.S. military has even used chlorine dioxide to sterilize medical equipment and electronic items to treat patients on the front lines of the war on Ebola in West Africa. It has also shown historical use and effectiveness against the influenza A virus.
Activated Versus Stabilized
Some products use the term “stabilized” or “naturally activated” chlorine dioxide. However, this is not true activated chlorine dioxide and does not give the full benefits of chlorine dioxide. The compound “stabilized” or “naturally activated” chlorine dioxide is in fact sodium chlorite, a salt.
True chlorine dioxide, which is a gas, requires mixing a basic salt solution with an acid. This gives off the chlorine dioxide gas. This is the reason OraCare comes in a two-bottle system and must be mixed before each use. The stabilized compound is not the same as chlorine dioxide, nor does it have the same oxidizing properties. The oxidizing potential is much lower, and the compound is far less useful as a product in general. Sodium chlorite has some benefits but nowhere near those of activated chlorine dioxide; specifically, it does not kill viruses.
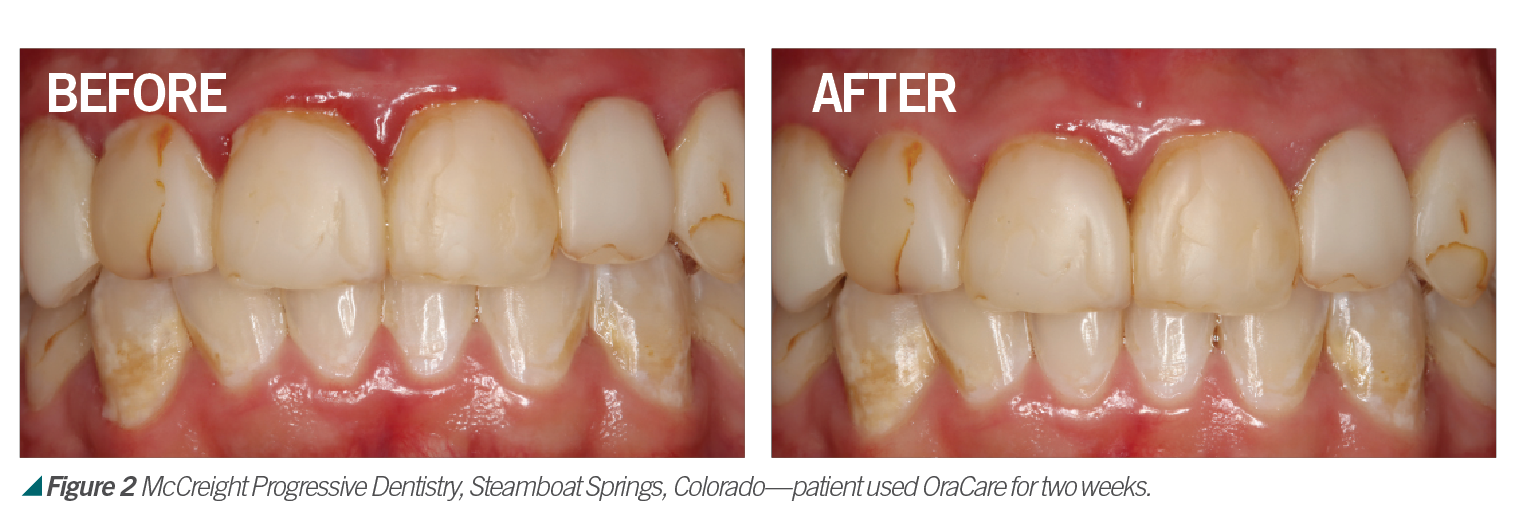
Chlorine Dioxide and Dentistry
Chlorine dioxide is having greater application in dentistry. It is used not only as an alternative to chlorhexidine, but also for implants, periodontal disease, bad breath, dry mouth, pre-rinsing, post-op, and more. Its ability to kill bacteria as effectively as chlorhexidine,2 without side effects, and additional benefits has brought dental professionals to a new level.
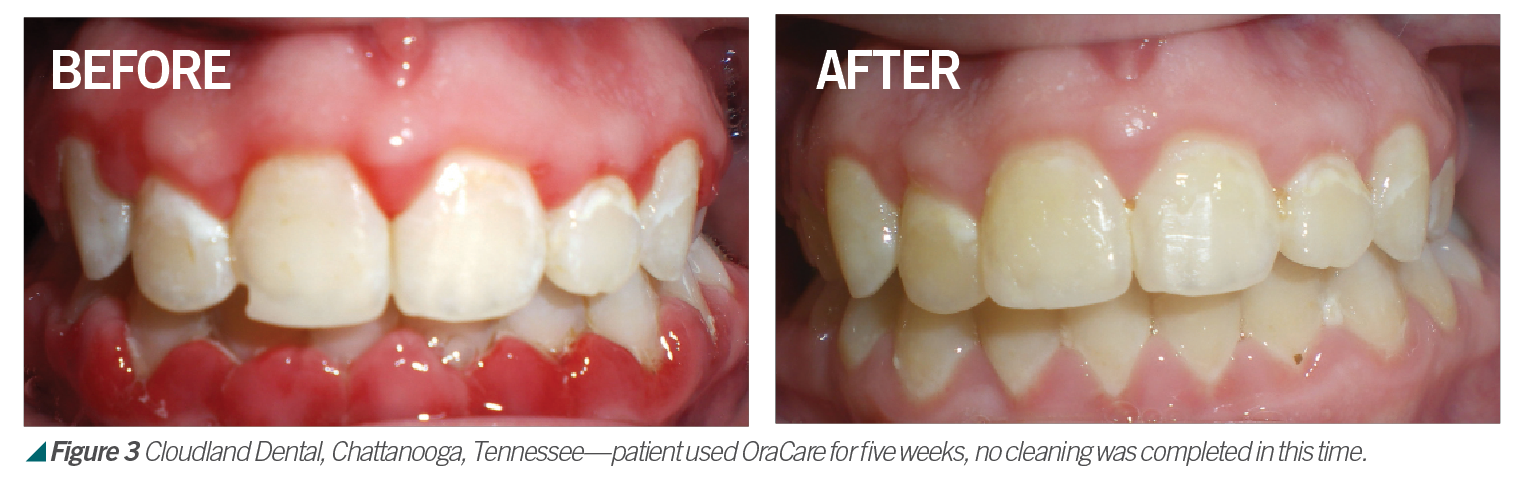
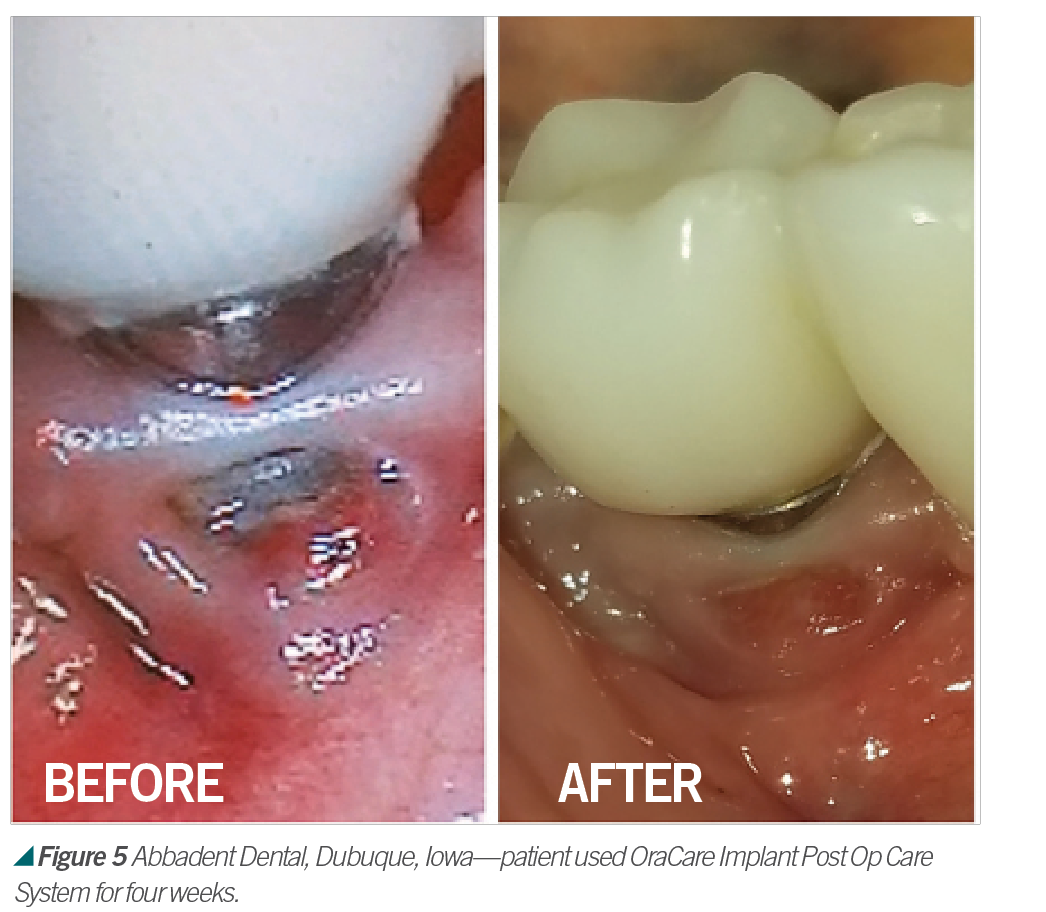
Activated chlorine dioxide meets all the profession’s needs and can provide protection daily in the office as a pre-rinse, especially with the raised concern regarding viruses. Activated chlorine dioxide can be found in OraCare products and is used in combination with xylitol. OraCare Health Rinse is exclusively sold in dental offices. Patients who have used OraCare have seen results such as the following cases: (see Figures 2-5).
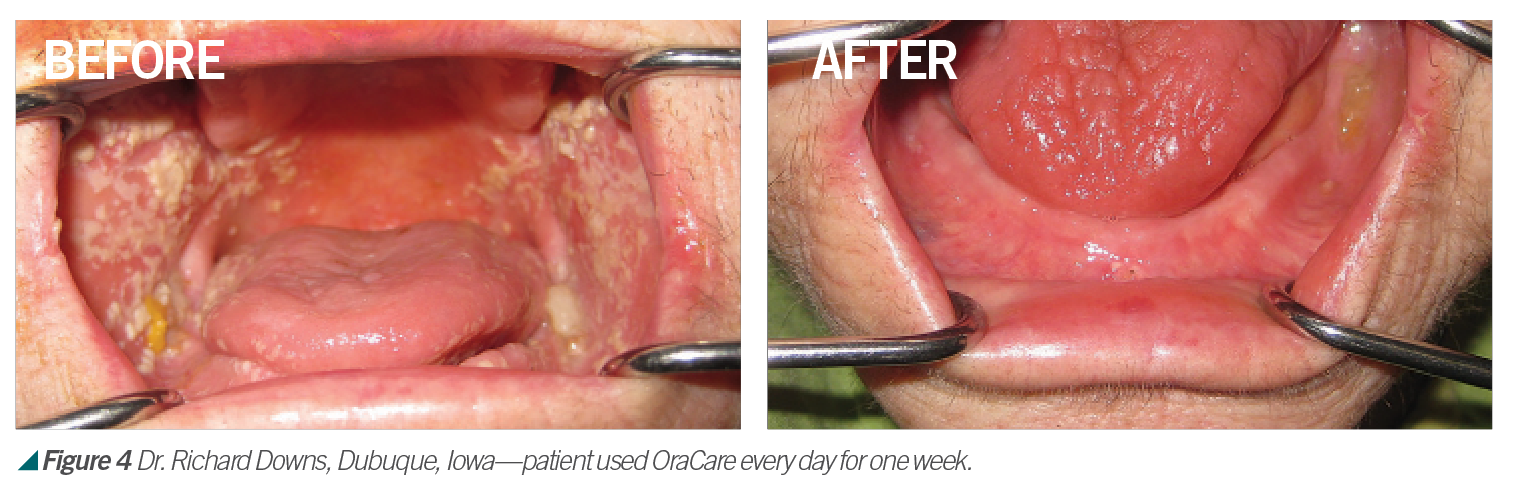
Dentistry has changed since 1954; so should the rinse being used. Because pre-rinsing has proved in many studies to reduce oral microbes before they become part of dental aerosols, it is important to consider using this new type of pre-procedural rinse. For more information, visit OraCareProducts.com or call 855-255-6722.
References
1. Alleyn CD, O’Neal RB, Strong SL, Scheidt MJ, Van Dyke TE, McPherson JC. The effect of chlorhexidine treatment of root surfaces on the attachment of human gingival fibroblasts in vitro. J Periodontol. 62(7):434-438. doi:10.1902/jop.1991.62.7.434
2. Downs RD, Banas JA, Zhu, M. An in vitro study comparing a two-part activated chlorine dioxide oral rinse to chlorhexidine. Perio-Implant Advisory. January 15, 2015. Accessed at https://www.perioimplantadvisory.com/clinical-tips/hygiene-techniques/article/16411500/an-in-vitro-study-comparing-a-twopart-activated-chlorine-dioxide-oral-rinse-to-chlorhexidine
3. Horner, C., Mawer, D., Wilcox, M., Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter?, Journal of Antimicrobial Chemotherapy, Volume 67, Issue 11, November 2012, Pages 2547– 2559, https://doi.org/10.1093/jac/dks284
4. “Chlorhexidine (Oral Route) Side Effects. “Mayo Clinic, Mayo Foundation for Medical Education and research, 1 Feb. 2020, www.mayoclinic.org/drugs-supplements/chlorhexidine- oral-rout/side-effects/drg-20068551?p=1.
5. “Chlorhexidine Facts.” Chlorhexidine Facts: Mechanism of Action, 2019, chlorhexidinefacts.com/mechanism-of-action.html.
6. Wyganowska-Swiatkowska, M., Kotwicka, M., Urbaniak, P., Nowak, A., Skrzypczak-Jankun, E., Jankun, J. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in
morphology. Int J Mol Med. 2016 Jun; 37(6): 1594–1600. Published online 2016 Apr doi: 10.3892/ijmm.2016.2550
7. Disinfectants chlorine dioxide. Lenntech. Accessed at https://www.lenntech.com/processes/disinfection/chemical/disinfectants-chlorine- dioxide.htm
8. Dewhirst, F., Chen, T., Izard, J., Paster, B., Tanner, A., Yu, W., Lakshmanan, A. and Wade, W. (2019). The Human Oral Microbiome. [online] Available at: https://jb.asm.org/content/192/19/5002 [Accessed 2 Feb. 2019].
9. FDA warns about rare but serious allergic reactions with the skin antiseptic chlorhexidine gluconate. U.S. Food & Drug Administration. 9 Feb.
10. 2017, https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety- communication-fda-warns-about-rare-serious-allergic-reactions-skin-antiseptic. Tsourounakis, I., Palaiologou Gallis, A. A., Stoute, D., Maney, P. and Lallier, T. E. Effect of Essential Oil and Chlorhexidine Mouthwashes on Gingival Fibroblast Survival and Migration, Journal of Periodontology, 84, 8, (1211-1220), (2013).
11. Krespi, Y. P., Shrime, M. G., Kacker A. The relationship between oral malodour and volatile sulfur compound-producing bacteria. Otolaryngol Head Neck Surg. 2006;135:671–6. [PubMed]
12. Kolahi J, Soolari A. Rinsing with chlorhexidine gluconate solution after brushing and flossing teeth: a systematic review of effectiveness. Quintessence Int. 2006;37(8):605–612.
13. Schmidt, J., Zyba, V., Jung, K, Rinke, R. Haak, R., Mausberg, R. F., and Ziebolz, D. Effects of octenidine mouthrinse on apoptosis and necrosis of human fibroblasts and epithelial cells – an in vitro study , Drug and Chemical
Toxicology, 10.1080/01480545.2017.1337124, 41, 2, (182-187), (2017).
14. Johnson PW, Yaegaki K, Tonzetich J. Effect of volatile thiol compounds on protein metabolism by human gingival fibroblast. J Periodontal Res. 1992;27:553–61. [PubMed]
15. Teixeira, K. I. R., Denadai, A. M. L., Sinisterra, R. D., and Cortés, M. E. Cyclodextrin modulates the cytotoxic effects of chlorhexidine on micro-organisms and cells in vitro , Drug Delivery, 10.3109/10717544.2013.879679, 22, 3, (444-453), (2014).
16. SaÄlam, M., Arslan, U.,Buket Bozkurt, Å, and Hakki, S. S. Boric Acid Irrigation as an Adjunct to Mechanical Periodontal Therapy in Patients With Chronic Periodontitis: A Randomized Clinical Trial, Journal of Periodontology, 84, 9, (1297-1308), (2013).
17. Ng, W., Tonzetich J. Effect of hydrogen sulfide and methyl mercaptan on the permeability of oral mucosa. J Dent Res. 1984;63:994–9. [PubMed]
18. “The Advantages and Disadvantages of Chlorhexidine Mouthwash.” Healthline. 1 Feb. 2020, https://www.healthline.com/health/chlorhexidine-mouthwash#side-effects.
19. Polimeni, G., Xiropaidis, A. V., and Wikesjö, U. M. (2006). Biology and Principles of Periodontal Wound Healing/Regeneration. Periodontology 2000, 41, 30-47. Retrieved from https://pdfs.semanticscholar.org/55c8/4509fcd32afafb67b16b34e46076f4b2d165. pdf.
20. Van Maanen-Schakel N. W., Slot D.E., Bakker E.W., Van der Weijden, G. A. The effect of an oxygenating agent on chlorhexidine-induced extrinsic tooth staining: a systematic review. Int J Dent Hyg. 2012;10(3):198–208. doi:10.1111/j.1601-5037.2012.00555.x
21. Van Strydonck D. A., Demoor, P., Timmerman, M. F., van der Velden, U., vander Weijden, G. A. The anti-plaque efficacy of a chlorhexidine mouthrinse used in combination with toothbrushing with dentifrice. J Clin Periodontol. 2004;31(8):691–695. doi:10.1111/j.1600-051X.2004.00546.
22. Wyganowska-Swiatkowska, M., Kotwicka, M., Urbaniak, P., Nowak, A., Skrzypczak-Jankun, E., and Jankun, J. (2016). Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. International Journal of Molecular Medicine, 37, 1594-1600. Retrieved from https://doi.org/10.3892/ijmm.2016.2550.

Floss & Flip Flops Episode 22: National Dental Hygiene Month
October 1st 2023Join the Sanders Sisters and Dr Anna Kay Thompson as they learn about the burning questions the medical community is not asking about oral health. Learn about all of the ways they are celebrating the good work of dental hygienists in their quest for whole-body health.
Maximizing Value: The Hidden Benefits of Preventing Hospital-Acquired Pneumonia Through Oral Hygiene
September 10th 2024Originally posted on Infection Control Today. Hospital-acquired pneumonia (HAP) is a significant infection prevention concern, leading to high patient mortality, increased health care costs, and ICU usage. Oral hygiene is an effective preventive measure.