Deep Dive: Ensuring the N95 Respirator Fits Right the First Time
A chat with HuFriedyGroup’s Senior Product Manager for Personal Protective Equipment Danny Forcucci on the importance of finding the right fit for N95s in dental practices.
Q: What is a HuFriedyGroup N95 Qualitative Fit Test Kit? Why do I need it for my dental practice?
The Occupational Safety and Health Administration (OSHA) requires that fit tests be conducted for those who need to wear respiratory protection, including N95 respirators, to ensure proper fit and protection. The HuFriedyGroup [N95] Qualitative Fit Test Kit provides the ability to perform pass/fail fit tests that assess the adequacy of respirator fit based on the individual’s response to a test agent. Because they can be administered in [the] office by existing staff, HuFriedyGroup N95 [Qualitative] Fit Test Kits provide a cost-effective way for employers to comply with OSHA requirements [29 CFR 1910.134].
Q: What comes in the kit? What does each item accomplish in the kit?
The HuFriedyGroup [N95] Qualitative Fit Test Kits come with all the components needed to perform fit tests according to OSHA-required protocols. Available with either Saccharin [sweet] or Bitrex [bitter] sensitivity and test solutions, each kit also includes a test hood, 2 nebulizers, replacement nozzles, cleaning tools, and a flash drive with detailed and easy-to-follow instructional materials. When donned, the hood creates a contained test environment. The nebulizers are used to aerosolize the solutions into the test hood with the sensitivity solution used to gauge sensitivity of the subject to the test substance and the fit testing solution used to conduct the test while the subject is wearing the respirator being validated for use. HuFriedyGroup [N95] Qualitative Fit Test Kits come with enough solution to conduct up to 55 tests, with additional refills, test hoods, and nebulizers available for purchase.
Q: When does an N95 need to be fit tested? Can’t I tell if it fits by the way it feels?
According to OSHA requirements, fit testing must be done before a specific brand, model, and size of a respirator is used for the first time. Additionally, annual testing should be conducted to ensure the respirator continues to fit the wearer properly over time. More frequent testing may also be required if the user experiences a significant change in weight or experiences other facial/dental alterations that may impact fit. Once a specific respirator is validated for use by an individual via a fit test, the user should perform a seal check with each donning thereafter. A user’s subjective feelings or seal check alone does not replace the need for annual fit testing.
Q: How does a fit test work? What are the steps? Is this something that will take a lot of time out of my busy day?
A qualitative N95 fit test relies on the respirator wearer’s senses to determine [whether] they smell or taste the aerosolized test substance while under the test hood. The OSHA-accepted fit test protocol requires first establishing that the subject can detect the test substance. Once established, the subject dons the respirator to be tested and performs a series of 8 exercises (eg, breathing deeply, turning head from side to side, reading a passage) for up to 60 seconds, during which the evaluator introduces the fit test solution at
Proper fit testing ensures that anyone who picks up an N95 respirator knows that it will fit, which means it will effectively protect the wearer from aerosol contaminants like COVID-19 causing pathogens. HuFriedyGroup’s test kits ensure best infection control.
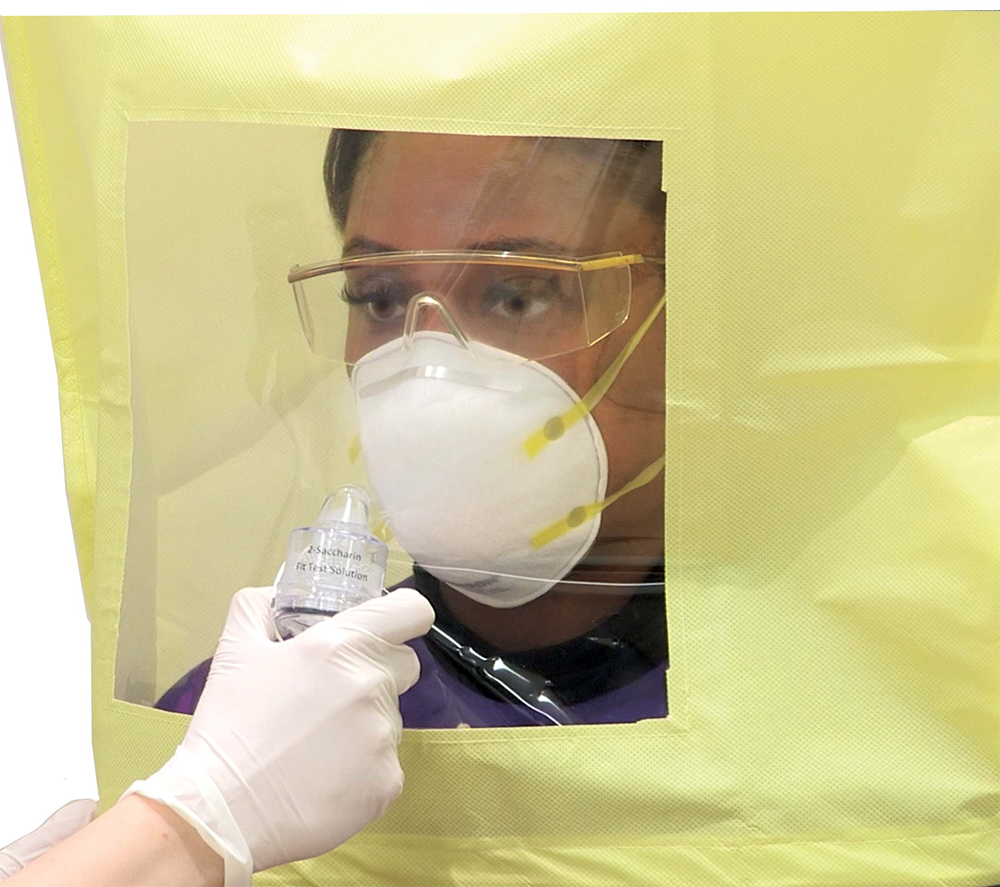
regular intervals through a slot in the test hood using the nebulizer. A failure is noted by the tester if the subject [can] sense the solution at any point during the test, and the testing stops. The sensing of the sweet [or bitter] aerosol during the testing indicates there is a gap in the seal of the respirator to the wearer’s face. [Although] the subject may attempt to readjust the respirator to achieve a better fit, the testing must be started from the beginning. The time necessary to conduct each individual test may vary but can generally be completed within [approximately] 15 minutes barring any failures. [Although] certification is not required to conduct a fit test, the individual conducting the fit test should thoroughly review the fit test kit instructions for use, including the test protocol and other instructional materials.
Q: Why is it so important to do a fit test?
Fit testing is important because it ensures the respirator being used by an individual is comfortable, fits well, and, most importantly, delivers the expected level of protection, minimizing the potential leakage of contaminants from the environment into the mask through the face seal.
Q: What is it about N95s that make them essential over other face masks?
Our Crosstex Isolator Surgical N95 Respirators are designed to achieve a tight seal around the nose and mouth of the wearer while providing a very efficient filtration of airborne particles. As surgical respirators, they are Class II medical devices regulated by the Federal Drug Administration and intended for use in health care settings to protect patients and clinicians from the transfer of microorganisms, body fluids, and particulate material. Unlike respirators, most surgical ear loop face masks are unable to achieve a tight fit around the wearer’s face, allowing some leakage of particulates into the mask via gaps around the face. [Although] they may provide some benefit—primarily in the form of source control—ear loop face masks [cannot] provide respiratory protection in the way N95 respirators [do].
Q: Each item in the fit test kit plays a different role. How long did it take to formulate what goes into this kit?
Appendix A of OSHA’s 29 CFR 1910.134 standard clearly outlines the OSHA-accepted fit test protocols [and] the equipment needed to adequately perform them. HuFriedyGroup [N95] Qualitative Fit Test Kits include the test components [and] the instructional material that allow employers to adhere to OSHA protocols for qualitative fit testing. These evidence-backed OSHA protocols have been well recognized and successfully administered for decades.
Q: What about if and when the COVID-19 pandemic slows? What makes these kits a smart investment for a dental practice?
After over 2 years, COVID-19 transmission levels continue to be high throughout the country. [Although] vaccines have proven effective at reducing the severity of the disease, respiratory disease prevention will need to be the ongoing focus in the face of COVID-19, its many variants, and new respiratory diseases that may hit us in the future. For this reason, it is increasingly important for dental practitioners to remain diligent, follow best practices, and take all measures available to them to protect themselves from respiratory infections, which includes the continued use of N95 respirators during aerosol-generating procedures.
These qualitative fit tests contain everything you need to make sure your N95 respirator is fit properly as per the standards set by the Occupational Safety and Health Administration. It also contains the instructional material to assist anyone in knowing exactly what each component is designed to do.

Surgical N95 respirators and fit testing go hand and hand in ensuring wearers receive the maximum possible level of protection from airborne respiratory hazards. Because a good and tight seal is difficult to determine, HuFriedyGroup [N95] Qualitative Fit Test Kits provide an easy-to-administer and cost-effective way to validate that the respirators your staff uses are effective. With constant changes in staffing, including differences in hiring and annual testing dates, qualitative fit test kits allow for ultimate flexibility. There is no travel required, scheduling conflicts, or the need for more expensive third-party testing options.
Q: Is there anything else you’d like to expand on?
Every individual is unique, and not all respirators fit every individual. In fact, the fit testing pass ratio of a particular respirator can vary quite broadly. Some may be better suited to larger face types, [whereas] others may not. For this reason, fit testing is a crucial component of every office’s respiratory protection program—whether N95 respirators are worn by all staff members every day with every patient, [only] during aerosol-generating procedures, or more infrequently.
Surgical N95 respirators and surgical ear loop face masks are single-use medical devices. [The Centers for Disease Control and Prevention] standard precautions call for clinicians to change out their respirators and masks with every new patient contact to minimize the risk of cross-contamination. A simple reminder is that the respirator or mask should be changed every time gloves are changed.
