Decontamination of N95 Respirators
CPAC Equipment recently began offering the RH-N95 Decontamination System to help the healthcare industry overcome and alleviate their N95 mask shortages.

Information provided by CPAC Equipment, Inc.
During these trying times, one common obstacle has been a shortage of personal protection equipment (PPE), including the medical-grade N95 masks needed by healthcare providers and first responders.
That’s why it was a big deal when CPAC Equipment, Inc., recently began offering the RH-N95 Decontamination System to help the healthcare industry overcome and alleviate their N95 mask shortages.
The use of High-Velocity Hot Air (HVHA) technology has been investigated as a mechanism to decontaminate N95 respirators and masks for re-use during their severe shortage caused by the COVID-19 pandemic. Published reports have demonstrated that dry heat processing is the preferred method to assure that both viral inactivation and N95 respirator performance efficacies are maintained for numerous re-use cycles.
Because of this, CPAC Equipment has repurposed its RH-Pro11 medical/dental sterilizer for N95 mask reprocessing, developing a dedicated decontamination cycle based on documented time-temperature parameters necessary to inactivate coronavirus without interfering with mask filtration and breathability performance for up to 20 re-uses.
This countertop unit can process approximately 100 masks per hour and can be located anywhere within a dental clinic or hospital, requiring only 110-115/12A service for its operation. The RH-N95 Decontamination System meets the FDA criteria for emergency use authorization during this crisis, with permanent 510(k) status to follow upon completion of microbial kill efficacy testing.
Regulatory Authority
The COVID-19 pandemic has induced critical shortages of N95 masks which are used as the primary first line of defense to stop the virus from entering the respiratory system and manifesting disease. The world was not prepared for the magnitude of this crisis, resulting in the use of inadequate masking or the attempt to decontaminate a previously used mask. For the latter to be successful in the decontamination process, the mask must be assured to have met the criteria for coronavirus destruction and for the maintenance of the mask’s original performance standards.
The FDA has recently released its guidance document entitled “Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency” (FDA1), which provides the guidance to provide the necessary safeguards of devices for mask re-processing. Meeting these FDA recommendations will allow devices not yet receiving the usually required FDA clearances to enter the market safely to relieve the N95 shortage. FDA emergency use authorization only grants authorization for previously cleared sterilization and disinfection devices which have been temporarily repurposed for decontaminating N95 respirators. No authorization has been granted by the FDA for devices that are used in the control of hospital acquired infections (HAI’s) such as UV room disinfectant units or other repurposed devices or technologies that have not been issued a 510(k) as a sterilization or disinfection device.
The CPAC RH-N95 meets those criteria, allowing the unit to enter the market under the FDA’s emergency authorization until final quantitation testing is complete for the issuance of an FDA 510(k). Until that work is completed, recognized (peer-reviewed literature) decontamination parameters for coronavirus inactivation and Mycobacterium inactivation allow the use of the RH-N95 as a decontamination device. This decontamination technology does not introduce any chemicals to the mask, nor does it affect the mask’s performance for breathability and filtration prescribed for the N95 mask performance by ASTM.
High-Velocity Hot Air RapidHeat
The CPAC Equipment RH-Pro11, which has been repurposed to the RH-N95, differs from the traditional dry heat sterilizer in which air remains static (air movement only by gravity convection) or in which air is minimally re-circulated by mechanical convection to enhance heat distribution. High-velocity hot air technology employs directed, uniform high-velocity hot air across the surface of a medical device which enhances heat transfer. As a heat conduction process, this thermodynamic mechanism heats and sterilizes/decontaminates both the surface and the solid interior of the device, including residues that may still be retained. The result is a marked reduction in time required for medical device sterilization or decontamination from hours to minutes by traditional dry heat sterilization.
The original Cox RapidHeat™ Transfer sterilizer serves as the predicate device to the RH-Pro11 and was granted 510(k) status from the FDA as a Class II (Performance Standards) device issued under K872643A and K881371. The RH-Pro11 sterilizer has been designed to sterilize medical instruments at temperatures of 375°F for 6-minute (unwrapped) and 12-minute (wrapped) cycles. High-velocity hot air sterilization has since been recognized and validated for use in healthcare applications including medical and dental offices, laboratories, ambulatory care clinics, and hospitals by the Centers for Disease Control and Prevention in their publications “Guidelines for Infection Control in Dental Health-Care Settings-2003” and “Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008.” Standards for use and process validation have been issued under the auspices of the American National Standards Institute and the Association for the Advancement of Medical Instrumentation in standards ANSI/AAMI ST40:2004(R)2010 and ANSI/AAMI ST50:2004(R)2018.
The high-velocity hot air process eliminates issues otherwise found in other thermal, chemical, or UV decontamination methods. Treatment mechanisms that introduce liquids to the mask run the high risk of severely lowering the mask’s filtration and breathability performance (Liao). This is especially true of water or water vapor that destroys the electrostatic nature of the mask’s filtration fabric which gives it the ability to meet its stated performance level. This would include steam sterilization and any liquid disinfection method. Chemical liquid/gas phase disinfection/sterilization technologies also introduce issues with:
- Incompatibilities of mask materials with the disinfectant/sterilant (includes electrostatic performance, band elasticity; adhesive performance, and metal reactions)
- Providing uniform concentration of the introduced (and maintenance of) required disinfectant/sterilant concentration
- Providing uniform mask penetration
- Mask wetness and required drying times
- Mask fiber matting and required filtration and breathability performance
- Toxic chemical residue retention and the ability to eliminate residues and monitor their removal
- Mask deformation
UV is not reliable due to its inability to penetrate the deep mask fibers and the “shadowing” interferences from mask fibers and dust residues that preclude the UV from contacting pathogens. No FDA-cleared UV sterilizers/disinfectors are known to be available, except for their use in mitigating hospital-acquired infections.
High-velocity Hot Air technology provided through the RH-N95 offers a simple mechanism that provides a relatively low, uniform temperature (155°F; 68°C) and a moderate exposure time of 30 minutes to decontaminate masks from coronavirus and other pathogens. Documented, peer-reviewed literature offers ample guidance in the temperature and exposure time required to inactivate coronavirus and other pathogens. No drying or residue removal is required of the process and at the relatively low temperatures required, little or no change in mask performance is expected.
Documented Parameters
Data from peer-reviewed literature provides a strong indication of those temperatures and exposures that are prescribed for the RH-95 can inactivate coronavirus and will maintain structural integrity and performance of the re-processed N95 respirator. This data has been instrumental in the construction of a decontamination cycle that should provide > 6 Log10 reduction (>99.9999 percent reduction) as demonstrated by Rabenau et al. (See references for additional information) for SARS-Co-V and Mycobacteria (Sung and Collins) at 60°C for 30 minutes. Rabenau et al. noted that heat treatment at 56°C for 30 minutes reduced a >7 Log10 SARS virus concentration to below the detection limit. Processing at 60°C for 30 min resulted in no infectious virus remaining (>7 Log10 reduction), regardless of the presence of any protein.
This data is also consistent with data provided by the World Health Organization for coronavirus (SARS) demonstrating an extrapolated 6 Log10 reduction in 22.5 minutes at 56°C (133°F). The data gleaned from these references leads us to believe that temperatures between 65-72°C (149-162°F) are adequate to fully inactivate the coronavirus (>6 Log10 reduction) at an exposure of 30 minutes without damage to mask components or to the mask’s performance. Although these microbial concentrations would seldom or ever be seen on a mask, these reduction levels provide the margin of safety necessary for mask re-use (FDA).
The studies presented above come from observations with liquid viral suspensions heated to temperature with various exposure times. However in a study by McDevitt et al. with inactivation of H1N1 virus on glass and stainless steel surfaces, it was demonstrated that with dry heat temperatures of 60°C and 65°C, this virus could be inactivated to the limits of detection at >5 Logs at 30 minutes exposure. This inactivation level is above the threshold required presently by FDA of > 3 Log10. This data supports the time-temperature parameters seen in the previously stated studies and supports the re-processing time and temperature prescribed for the N95 mask cycle.
The FDA is requiring that Mycobacterium inactivation be assessed, recommending a >6 Log10 reduction. Mycobacterium represents the most heat resistance of microbial species (other than spores and prions) and is used as the demonstrative surrogate for inactivation of other vegetative bacteria and of fungi, parasites, viruses, and Mycobacterium. The demonstration of >6 Log10 reduction of Mycobacterium would further assure user safety from the destruction of other pathogens that might accompany a used mask.
Literature searches have demonstrated that Mycobacterium paratuberculosis, can be inactivated in solution to a >6 Log10 reduction in 6 minutes at 65°C (149°F) and at 68°F (155°C) in 2.2 minutes. Mycobacterium paratuberculosis is more thermally resistant than M. bovis, the heat-resistant surrogate designated for Mycobacterium challenge inactivation studies. The time-temperature profiles selected for N95 mask re-processing exceed those profiles required to inactivate >6 Log10 reduction of Mycobacterium.
For completion of the requirements necessary for an FDA-clearance, testing will be conducted using a coronavirus surrogate such as a Murine Hepatitis Virus or Human Coronavirus 229-E to assure the coronavirus is inactivated by >3 Log10 (FDA1,2). Additionally, testing will be conducted to assure a reduction of >6 Log10 can be achieved on a Mycobacterium species to denote the destruction of other microbiological pathogens that may have also contaminated the mask during its use. These studies will be initiated shortly to meet FDA 510(k) marketing clearance requirements.
Mask Performance Analysis
Inherent for the re-use of an N95 mask is the requirement to assure that the decontamination process does not jeopardize the safety and health of the healthcare practitioner re-using the N95 mask. N95 respirators/masks are commonly made of nonwoven fabric consisting of three composite layers: Outer-layer consisting of spunbond polypropylene; mid-layer consisting of meltblown polypropylene; and an inner-layer consisting of spunbond polypropylene). Some N95 respirators also incorporate an additional layer of cellulose/polyester nonwoven, the outer active layer coated with a hydrophilic plastic laminated to a second inner layer treated with copper and zinc ions. This additional dual layer is designed to inactivate influenza viruses using deferent mechanisms of action. Those masks containing this additional inactivation layer containing cellulose is incompatible with hydrogen peroxide re-processing technologies.
The polypropylene, polyester, and cellulose components all are compatible with the HVHA process employed in the RH-N95, including the retention of the electrostatic charge in the polyethylene layers. Typically, the other components of N95 masks are also thermally compatible with the lower temperatures associated with the HVHA process: (e.g., straps composed of thermoplastic elastomers; nose clip made from aluminum; nose foam derived from polyurethane; and thermally resistant adhesives).
Specification performance of the mask’s integrity, composition, filtration and breathability are physical parameters that require analysis after the mask has been re-processed (FDA2). All visible indications from testing conducted by CPAC demonstrate the masks have not been altered by the decontamination process after twenty 30-minute re-processing cycles, the limit of the number of cycles conducted to date.
Observations are consistent with a report from Stanford University, which reported that after the N95 filtration fabric was dry heat-treated at 75°C for 30 minutes after 20 cycles there was no change in filtration efficacy or pressure drop. Whole mask studies after 20 cycles at this time and temperature also showed no deformations in the mask or changes in elasticity of face/ear straps (Liao). To demonstrate the maintenance of the recognized performance testing for filtration efficacy and breathability, masks undergoing 20 re-processing cycles will be tested against ASTM Standard criteria under F2100 in accordance with documentation required for 510(k) FDA-clearance.
RH-N95 Capabilities
The RH-N95 consists of four trays each capable of holding 12 N95 masks (nested, double-stacked), allowing ninety-six N95 masks to be processed in just over an hour. The decontamination cycle time for N95 decontamination is presently set at 30 minutes at a temperature of 68°C (155°F) in accordance with the parameters gleaned from the literature cited above. Total processing time is 34 minutes to allow for mask pre-heating before the decontamination cycle initiates. There is no water used in the process so the masks are dry at retrieval. The unit runs on 110-115V, 12 amps or 220-230V, 6 amps. It is a countertop unit and requires no other utilities, allowing mask processing to occur department-by-department or floor-by-floor.
Figure 1. Time-Temperature Profile of Nested N95 Masks Within an RH-Pro11 N95.
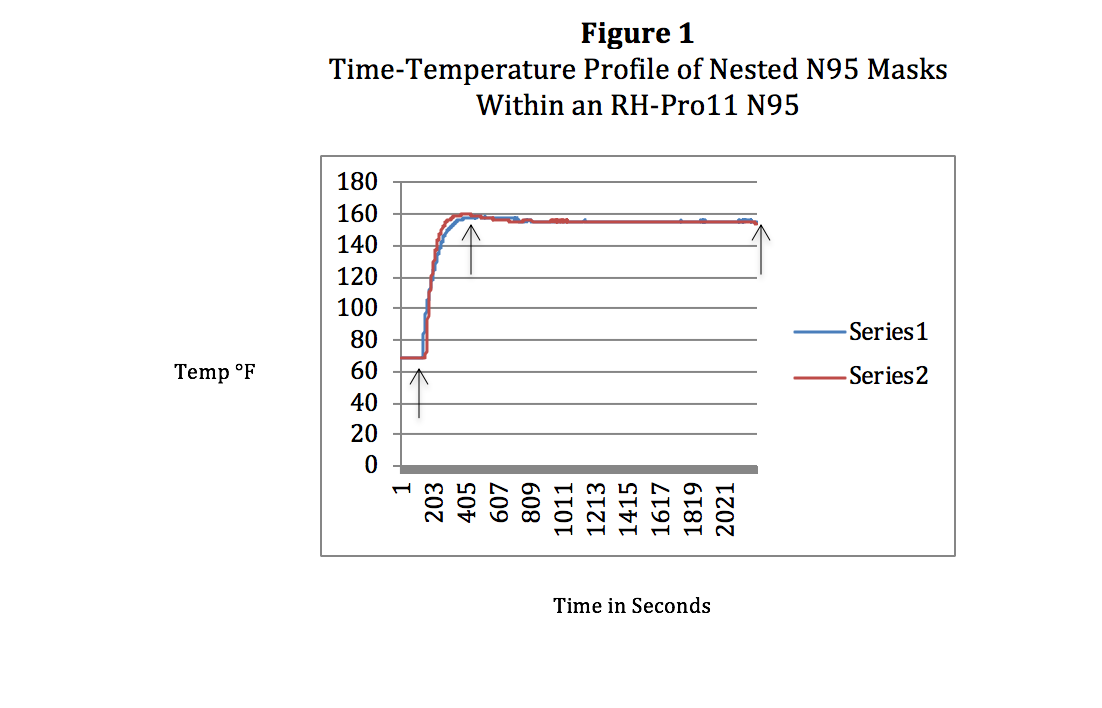
The RH-Pro11 has been reconfigured to the RH-N95 to accommodate these mask-required re-processing temperatures of 155-156°F. Changes in software have enabled CPAC to create a specific cycle for mask decontamination. The graph in Figure 1 is thermocouple temperature data (Labjack T7-Pro, T-Type thermocouples) derived from two N95 3M 8210 masks nested together under full load conditions, one thermocouple on the outside mask (Series 2) and the second on the inside mask (Series 1). Arrows indicate Process Start (left), Decontamination Cycle Initiation (middle), and Process Finish (right).
As seen in Figure 1 these cycle parameters meet all conditions that allow the masks to retain its specifications and function and provide the necessary viral kill levels to assure mask reliability and safety in its re-use. Temperatures do not exceed 69°C (156°F) with a temperature range of 155-156°F from 4 minutes into the cycle to completion of the 30-minute decontamination cycle (total 34 minutes from start of processing to finish). These time-temperature profiles are identical to single, non-nested masks, assuring nested masks present no barrier to high-velocity hot air technology.
These conditions will allow the RH-N95 to process up to 48 masks per 30-minute cycle or approximately 100 masks per hour, enabling healthcare facilities to meet the demand for N95 masks placed on them by this epidemic. Two models of N95 masks have been tested for repetitive re-processing at this time: 3M N95 8210 and Kimberly-Clark Corporation PFR95. It should be further noted that by visual inspection, no heat incompatibilities were found through 20 cycles of re-processing in the elastic bands, foam nose fittings, and adhesives to detract from secure and form fit to nose and face.
We have demonstrated that an approach to replacing CIs with the CIR does provide the time and temperature data far superior to that of a CI. The CIR is placed onto a tray within the RH-N95 unit, generating the graph shown in Figure 2. Tabular data is also generated and computer stored. Data retrieved is within one degree Fahrenheit of that data retrieved from the Labjack thermocouple data (described previously) once the CIR data logger has had the opportunity to come to temperature about eight minutes into the cycle.
Figure 2. CIR Generated Data of N95 Decontamination Cycle.
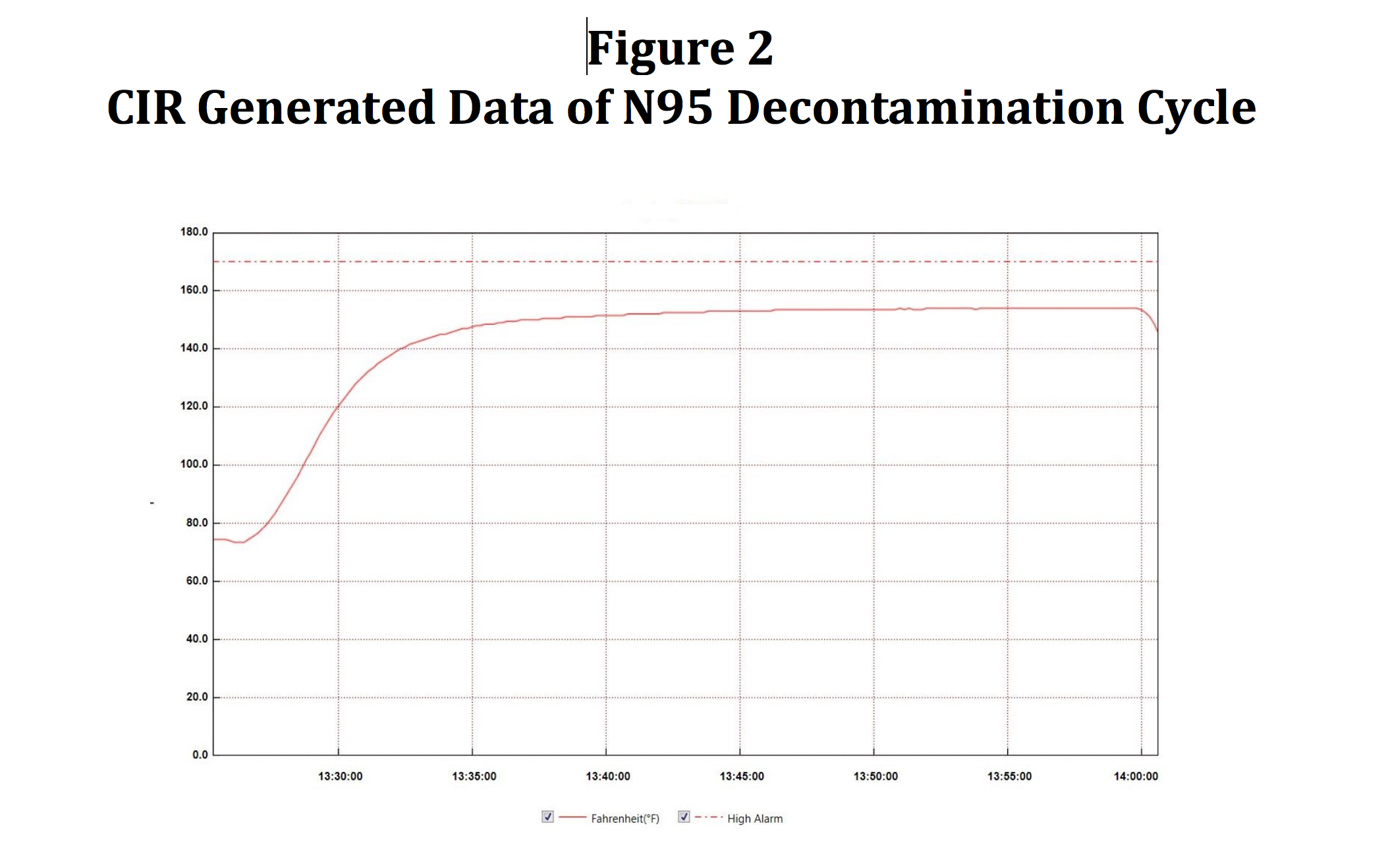
It should be noted that the time required for decontamination of the N95 masks in the RH-N95 may be significantly lower than the 30-minute exposure time derived from the literature. HVHA technology employed in the RH-Pro series of sterilizers has been demonstrated to reduce exposure times by 80% when compared to traditional static (non-moving) air of dry heat sterilizers or dry heat ovens. Initial microbial inactivation studies by CPAC will use parameters found in the literature for its initial cycle settings. However, investigations will continue to determine if lower exposure times can provide a more expedited decontamination process.
Summary
The RH-N95 has been allowed to begin marketing under the recent FDA Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers During the Coronavirus 2019 Public Health Emergency document (FDA1). Based on documented evidence that coronavirus can be inactivated at a >5Log10 reduction at 155-156°F with an exposure of 30 minutes, a dedicated N95 mask treatment cycle was created for RH-N95. The time-temperature parameters utilized appear to cause no physical damage or inhibit mask filtration/breathability performance. Studies will soon be underway to quantify viral inactivation and mask performance in accordance with FDA 510(k) marketing clearance requirements.
About the Author
Nelson S. Slavik is Senior Microbiologist and Vice President of Research and Development, CPAC Equipment, Inc. Responsibilities include research and development of infection control, patient safety, and sterilization technologies. Academically, he holds dual degrees from the University of Illinois at Urbana-Champaign, which include a Ph.D. in Microbiology and a Master of Science in Biochemistry/Microbiology. He has consulted to the American Hospital Association as their primary environmental regulatory consultant for over 10 years providing testimony to Congress and providing workshop presentations nationally and internationally. He has authored over 80 articles on environmental/occupational safety and infection control, participating in over 100 workshops and seminars. He holds two patents on High-Velocity Hot Air technology.
References
- Centers for Disease Control and Prevention. Required information for effective infectious disease outbreak response SARS-Cov-2 (Covid-19) Updated 3/4/2020. https://www.dhs.gov/sites/default/files/publications/2020_03_18_mql_covid-19-sars- cov-2_-_cleared_for_public_release_0.pdf
- FDA1, Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-sterilizers-disinfectant-devices-and-air-purifiers-during-coronavirus-disease?utm_campaign=2020-03-29%20FDA%20Issues%20New%20Enforcement%20Policy%20for%20Sterilizers&utm_medium=email&utm_source=Eloqua
- FDA2, Enforcement Policy for Face Masks and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency: Guidance for Industry and Food and Drug Administration Staff, March 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-face-masks-and-respirators-during-coronavirus-disease-covid-19-public-health
- McDevitt, J et al., Role of Absolute Humidity in the Inactivation of Influenza Viruses on Stainless Steel Surfaces at Elevated Temperatures. Applied and Environmental Microbiology 76:12 (pgs. 3943-3946) 2010.
- Liao, L et al., Can N95 facial masks be used after disinfection? And for how many times? Report from the collaboration of Stanford University and 4C Air, Inc., March 25, 2020. https://stanfordmedicine.app.box.com/v/covid19-PPE-1-2
- Rabenau, HF, et al., Stability and inactivation of SARS coronavirus. Med Microbiol Immunol 194: 1–6 (2005). https://www.researchgate.net/publication/8586211_Stability_and_inactivation_of_SARS_coronavirus
- Sung, N and Collins, MT, Thermal Tolerance of Mycobacterium paratuberculosis. Applied And Environmental Microbiology, Mar: 999–1005, 1998. https://aem.asm.org/content/64/3/999
- World Health Organization. First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network. https://www.who.int/csr/sars/survival_2003_05_04/en/
Summary of CPAC Equipment’s HVHA RapidHeat™ RH-N95 Mask/Respirator Re-processor
Reconfigured from CPAC Equipment’s RH-Pro series of FDA-cleared sterilizers, the RapidHeat RH-N95 Mask/Respirator Re-processor has been designed to decontaminate N95 respirators, to counteract the severe shortage of N95 masks during this pandemic. The RH-N95 offers the following features/advantages over other thermal, chemical, and radiological alternatives as listed below.

RH-N95 Features
- Preferred decontamination process (Stanford Report, March 2020), that provides documented evidence of maintaining filtration and breathability performance (no electrostatic charge reduction in meltblown fibers used as viral filtration fabric)
- Meets or exceeds published (peer-reviewed) >6 Log10 reduction requirements for coronavirus and Mycobacterium to assure coronavirus kill and that of other non-spore pathogens as well
- Meets FDA criteria to market device for coronavirus decontamination under FDA’s emergency authorization
- Dedicated unit for the re-processing of N95 respirators/masks
- Requires no water in its operation; requires only 110-115V/220-240V electric service that is commonly available
- No ancillary chemical or other treatment or supply products required
- No drying time required after treatment; masks are cool to the touch in seconds after their removal from the unit and ready for immediate re-use
- No residues to evacuate from treated masks after treatment; no worry about lingering toxic chemical residues to sensitive nasal passages, facial skin, and other respiratory passages
- Ability to monitor decontamination parameters (time-temperature). Other non-thermal processes have no method to measure chemical concentrations at site of required kill
- No deformation in mask structure or change in filtration/breathability performance for at least twenty (20) re-use cycles
- Low cost for RH-95 Mask Re-Processor (~ $7,500)
- Multi-purpose unit that functions as N95 mask re-processor, but can be simply re-programed via USB port to be used as a medical/dental sterilizer (FDA-cleared) post-pandemic
- Countertop unit with small footprint which can be placed almost anywhere electrical service is available
- Four-tray configuration allowing approximately 100 masks to be processed per hour
CPAC Equipment, Inc.
800-828-6011 | cpac.com