A new weapon in the fight against recurrent caries
Researchers are making tremendous progress in formulating caries-resistant composite resins.

It is impossible not to love the vast shade palette, versatile application and minimally invasive preparation design made possible by composite resin restorative materials. You and many of your colleagues have assuredly admired the beauty of a well-placed resin composite.
Fast-forward a few years and you may well be one of the thousands of dentists replacing those meticulously placed composite restorations secondary to recurrent decay. Over half of restorations placed by dentists in the U.S. are replacing failing restorations - the vast majority being of a composite resin material.1
Read more: 4 big benefits of resin cements
Even in patients with meticulous oral hygiene and essentially sugar-free diets, composite resins are inherently susceptible to degradation by not only cariogenic bacteria but also intrinsic enzymes of healthy human saliva.2 In the words of an amazing clinician, well-respected researcher and personal friend, “It should be eye opening that composites do not cure caries and are not perfect!” (Dr. Augusto Robles, University of Alabama at Birmingham School of Dentistry).
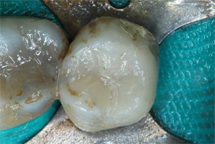
Posterior resin composite at time of placdement with highly polished surface.
While a true statement, researchers are making tremendous progress in formulating caries-resistant composite resins. The notion of “bioactive composite resins” has been circulating through the literature since the development of glass ionomer restoratives with a recent resurrection in this field. These composite resin formulations have been deemed capable of releasing fluoride, calcium, phosphorous, or a combination of these elements to aid in remineralizing decalcified areas of the tooth.3
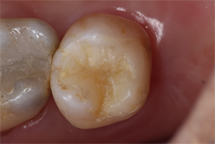
Same composite following six months of clinical service covered in a layer of plaque biofilm.
In recent years, oral healthcare has been making a transition away from reactionary care to preventative care. For such a transition to be successful, dental materials must also make such a shift. Though bioactive remineralizing dental materials are obviously useful, these agents are treating the signs of decay and not the etiology. Hence, anti-microbial composites have made an entrance into the world of dentistry.
Anti-microbial composites: What are they?
All dentists know of the bacterial biofilm that forms over the protective salivary pellicle in the early stages of plaque accumulation. In this cariogenic bacterial metropolis, adhesion proteins as well as acidic metabolites are deposited and the carious process continues.
Trending research: How scientists are turning teeth into drugs
Anti-microbial/anti-biofilm composite resins act to resist the carious process via:
1. Non-selectively killing bacteria contacting the restoration.
2. Selectively killing pathogenic bacteria contacting the restoration.
3. Repelling proteins necessary for bacterial attachment to the restoration.
Broad spectrum approach: non-selective bacterial destruction
Let’s begin with the heavy hitters – the agents proven to kill the majority of bacterial species found in the oral cavity. These include chlorhexidine, elemental silver and quaternary ammonium compounds.
Trending article: Making the transition to mercury-free dentistry
Chlorhexidine
Popular among the periodontal community, 0.12 percent chlorhexidine gluconate has made a name for itself as Periogard® oral rinse. Recent studies have aimed to encapsulate chlorhexidine molecules and mesoporous silica nanoparticles in composite resins to create an anti-bacterial powerhouse of a resin restoration. Chlorhexidine’s ability to destroy the outer and inner cell membranes and induce apoptosis is directly proportional to the concentration of the agent.4 More chlorhexidine leads to more bacterial destruction. Sounds great, right? The unresolved problem with this potential miracle-worker is its “burst-release kinetics” from the resin restoration.4 Large amounts of chlorhexidine are released in the first few days, leaving you with a regular old composite restoration after about a week – susceptible to the same destruction as the non-antibacterial resin composites.
Silver particles
The use of silver as an antibacterial agent predates chlorhexidine (some reports state we are in the sixth millennia of using silver as an antibacterial agent). Elemental silver inactivates bacterial enzymes responsible for synthesizing bacterial DNA replication.5 No DNA replication means no growth nor repair, which results in cell death. Nanoparticles of silver incorporated into composite resins have shown success in preventing microbial biofilm formation and inducing bacterial cell death without reducing bond strength nor discoloring the restoration.6 So, what’s the problem? Similar to chlorhexidine containing resins, these silver nanoparticles portray the dreaded burst-release from their resin matrix.
The common denominator of disadvantages for these antimicrobial resins appears to be the sudden massive release of the antimicrobial agent. How do we prevent this from occurring? Maybe we should try covalently bonding the antimicrobial agent to the resin matrix.
Quaternary ammonium methacrylate compounds
Similar to the chlorhexidine products, quaternary ammonium compounds (QAMs) have a positive track record among the dental community. Cetylpyridinium chloride is a QAM found in many “anti-plaque” and “intense germ-killing” oral rinses. QAMs can covalently bind to the composite resin matrix and thus be slowly released as the restoration is subjected to enzymes of the oral cavity.7 The resulting decrease in biofilm formation and lactic acid concentration around QAM containing composite resins has been reported to remain consistent for greater than 12 months.
Read more: Using silver diamine fluoride as a new caries management solution
How do they work? QAMs have a net negative electromagnetic charge. The bacterial cell surface has a net positive electromagnetic charge. The negatively charged QAMs attach to the positively charged proteins on the bacterial cell surface and block the transport of water, ions and metabolic products across the bacterial cell membrane.8 If a sufficient number of transport proteins on the cell membrane are blocked, the bacterial cell dies via either accumulation of metabolic products inside the cell or increased intracellular osmotic pressure (the cell explodes). QAMs of various chain lengths have been researched. The most effective QAMs appear to be those of larger chain lengths (chain length of roughly 16).8 In other words, larger QAMs lead to better blockage.
Targeting the bad bugs: selective bacterial destruction
The rich and diverse oral microbiome consists of anywhere between 500 and 700 different bacterial species,9 most of which contribute to oral health. Relatively few of these hundreds of species are responsible for diseases of the oral cavity. To promote oral health, the ideal antimicrobial agent will kill and/or prevent proliferation of disease-causing bacteria and allow protective bacteria to take over the oral niche.
This is where we introduce the quaternary ammonium compound dimethylaminododcyl methacrylate (DMADDM). Recent studies incorporating DMADDM into composite resins have reported decreased numbers of streptococcus mutans (the No. 1 bacteria associated with caries) and streptococcus sanguinis alongside unchanged numbers of streptococcus gordonii (bacteria associated with sound enamel structure).9
Trending article: 5 ways that traditional impressions can go wrong
Though the reasoning behind this finding of increased susceptibility of certain streptococcal species to DMADDM has not yet been elucidated, such an agent shows potential for effective utilization in anti-biofilm composite resins.
Though QAM-containing composite resins show enormous promise, their ability to induce bacterial cell destruction is directly related to contact occurring between the QAM-containing resin and the bacterial cells. In the oral environment, a uniform protein coating develops over the surface of the tooth and serves as a barrier between bacteria and the QAM containing resin composite. If the QAMs are unable to directly bind to the bacterial cell surface, bacterial cell destruction cannot occur.
Bacteria cannot destroy that which they cannot touch
Protein-repelling agents
The most prevalent protein-repelling biomimetic material among the medical and dental community is a mouth-full: 2-methacryloyloxyethyl phosphorylcholine (“MPC” for short). Joint replacement prosthesis, coronary stents and other medical devices with MPC coatings have gained FDA approval and are regularly utilized in clinical settings. The polymerization of this zwitterionic phosphorylcholine moiety-containing custom methacrylate10 (say that five times fast) with the conventional resin composite matrix has allowed for the creation of timed-release, protein-repellant composite resin materials.
How does it work? MPC is a hydrophilic molecule, meaning it attracts water. A completely hydrated MPC polymer contains a significant amount of free circulating water particles capable of binding to any nearby protein molecules. Instead of the protein molecules binding to the resin material, they bind to these circulating free water molecules and are readily cleared from the resin surface.
Though MPC has not been shown to directly kill bacterial cells, the sustained release and ability to prevent bacterial binding to the restoration surface makes its incorporation into dental composites one of the most promising antimicrobial agents.11
Small molecule inhibitors
Another approach to prevent biofilm attachment is targeting the enzymes that streptococcus mutans uses to produce “sticky” glucan polymers. These glucan polymers help attach the plaque biofilm to the tooth. Small molecules have been identified that inhibit these enzymes.12 Rat models have shown that application of a solution of this small molecule to teeth can reduce the incidence of caries. A next step would be to incorporate these small molecules into a composite resin. An advantage of this approach is that it would prevent tooth demineralization without significantly altering the intraoral microbial flora.
Read more: Study finds virulent bacteria linked to caries in children
We have the technology, now we need to get it into the clinic
These emerging technologies are all promising solutions to create a resin composite that will resist its biggest threat: acid-producing bacterial plaque. To the best of the knowledge of these authors, there is not yet a commercially available restorative material utilizing any of these approaches. A scan through the abstracts of the most recent American Association for Dental Research meeting shows that these technologies are starting to translate from concept to reality. With time for product development and clinical evaluation, hopefully these materials will arrive in our clinics soon.
References
1. Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. 2001. Quality of dental restorations: FDI Commission project 2-95. Int Dent J. 51(3):117-158
2. Finer Y, Santerre J. 2004. Salivary esterase activity and its association with the biodegradation of dental composites. J Dent Res. 83(1):22-26
3. Xu H, Moreau J, Sun L, Chow L. 2011. Nanocomposite containing amorphous calciums phosphate nanoparticles for caries inhibition. Dent Mater. 27(8):762-769.
4. Zhang J, Wu R, Fan Y, Liao S, Wang Y, Wen Z, Xu X. 2014. Antibacterial dental composites with chlorhexidine and mesoporous silica. J Dent Res. 93(12):1283-1289.
5. Morones J, Elechiguerra J, Camacho A, Holt K, Kouri J, Ramirez J, Yacaman M. 2005. The bactericidal effect of silver nanoparticles. Nanotechnology. 16(10):2346-2353.
6. Cheng Y, Zeiger D, Howarter J, Zhang X, Lin N, Antonucci J, Lin-Gibson S. 2011. In situ formation of silver nanoparticles in photocrosslinking polymers. J Biomed Mater Res B Appl Biomater. 97(1):124-131.
7. Imazato S, Chen J, Ma S, Izutani N, Li F. 2012. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jap Dent Sci Rev. 48(2):115-125.
8. Li F, Weir M, Xu H. 2013. Effects of quaternary ammonium chain length on antibacterial bonding agents. J Dent Res. 92(10):932-938.
9. Wang S, Zhang K, Zhou X, Xu N, Xu H, Weir M, Ge Y, Wang S, Li M, Li Y, et al. 2014. Antibacterial effect of dental adhesive containing dimethyl-aminododecyl methacrylate on the development of Streptococcus mutans biofilm. Int J Mol Sci. 15(7):12791-12806.
10. Lewis A. 2000. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf B Biointerfaces. 18(3-4):261-275.
11. Cheng G, Zhang Z, Chen S, Bryers J, Jiang S. 2007. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials. 28(29):4192-4199.
12. Zhang Q, Nijampatnam B, Hua Z, Nguyen T, Zou J, Cai X, Michalek SM, Velu SE, Wu H. 2017. Structure-Based Discovery of Small Molecule Inhibitors of Cariogenic Virulence. Sci Rep. 20;7(1):5974.