Keystone Industries receives FDA clearance for Keysplint Soft
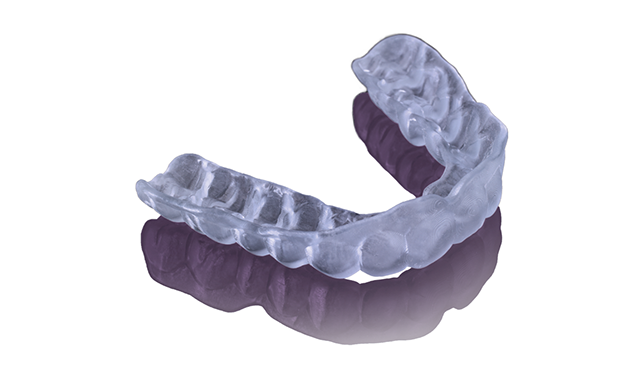
Keystone Industries® has received 510k clearance from the FDA to sell its 3D printing night guard and splint resin in the US, the company announced yesterday in a press release. The US now joins Canada and the European Union, where KeySplint Soft is already available.
The KeySplint Soft is said to be unique among 3D printing materials, offering a tough and durable hard splint that can withstand the forces of bruxism but is still flexible enough for patient comfort.
Manufactured in the US, the KeySplint is designed to be abrasion and fracture-resistant with a guaranteed shelf life of three years. It will be available in two shades-a clear version that is optimized specifically for the Carbon® Digital Manufacturing Platform and an open-source version with a violet tint, the press release said.
“This is a significant event in that the FDA has not reviewed and cleared many 3D printing materials as medical devices, and KeySplint Soft is believed to be just the second FDA clearance for splint and night guard applications,” said Ira Rosenau, president of Keystone’s dental group. “In order to compliantly 3D print night guards or splints in the United States, users need to look for materials that have undergone and cleared the FDA 510k process. We are pleased to be one of the first compliant splint solutions here in the USA.”
The combination of the KeySplint Soft Clear resin and Carbon’s M series printers allows labs to produce six times more splints than traditional production methods, Keystone said in their statement. This is said to increase profit per unit by nearly 75 percent.
Keystone has validated KeySplint Soft in several printers and with several post-cure units, including from Carbon, Asiga, MiiCraft, and others, with additional validations ongoing, the statement said.
Keystone’s group of dental companies focus on consumable laboratory products and operatory/preventative products. Keystone is a global supplier with a network of more than 800 US and international distribution partners in over 70 countries. For more information, visit keystoneindustries.com.