Urbanek TMJ Device is Designed to Relieve the Inflammation that Causes Temporomandibular Disorder
Company announces the launch of commercial operations in North America; now offers solution for TMD/TMJ sufferers nationwide.
A Nashville, Tennessee oral maxillofacial surgeon who has been effectively helping eliminate temporomandibular disorder (TMD/TMJ) is now launching commercial operations to help many more patients, often in as few as 2 office visits.
The Urbanek TMJ Device and Protocol—designed by Anthony Urbanek, DDS, MD, MS—this week announced an official launch for its commercial operations in North America, offering dentists a new way to treat the 10+ million TMD/TMJ sufferers in the United States.
“After more than 4,000 cases treated with a 95% success rate, we’re excited to finally offer this solution nationwide to treat the growing epidemic of TMD. With this FDA-cleared medical device and our comprehensive training and support, dental care providers can now offer their patients an easy and affordable solution,” says CEO and Founder Dr Urbanek.
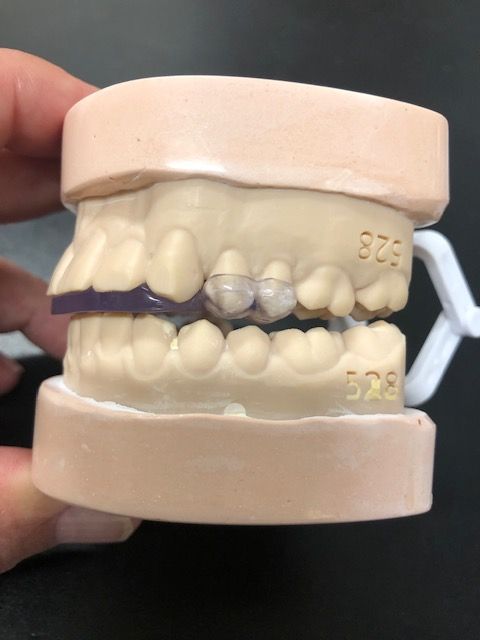
Developed by the oral maxillofacial surgeon with over 40 years of experience, the Urbanek Device and Protocol is the only non-surgical, custom CAD-CAM, 3D-printed resin device that alleviates the inflammation of the TMD joint. Unlike a nightguard, this FDA-cleared medical device was specifically designed to treat the root cause of TMD.
“It’s our mission to provide dental care professionals with a system to safely eliminate TMJ/TMD for their patients without surgery or medications,” says Dr Urbanek.
The device, customized to each patient, unloads the joint just like a set of crutches unloads an inflamed knee, the company says. The patient starts by wearing the device 24/7 for 2 months, and then at nighttime only for the rest of their life. That works for about 85% of the patients, reportedly, and the company has completed 4000 patient cases with a success rate of 95%, which indicates what can happen if they follow the protocol. Results have been successful with patients in as few as 2 office visits, the company states.
For more information,visit www.UrbanekTMJ.com or register for Dr Urbanek’s CE webinar here: https://bit.ly/3WHfb2W. Clinicians can take the 2.5-hour online course to become successful and safe with the basics of the method. After listening to the whole course and taking a short test for CE [continuing education] credits and certification, they receive certification to use the device.