Study finds new structure of cavity-causing bacteria
In a new study, researchers at the University of Pennslyvania School of Dental Medicine and the Georiga Institute of Technology found that microbes’ spatial organization is crucial to how they cause tooth decay.
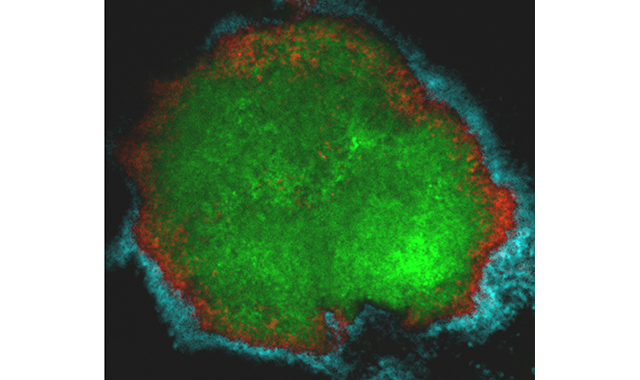
In a new study, researchers at the University of Pennsylvania School of Dental Medicine and the Georgia Institute of Technology found that microbes’ spatial organization is crucial to how they cause tooth decay. Published in the journal Proceedings of the National Academy of Sciences’ May 2020 edition, the study states that Streptococcus mutans, one of the major bacterial species responsible for tooth decay, is encased in a protective community of other bacteria, forming a spatial organization associated with the location of the disease onset.
While studying bacteria in a petri dish or test tube has led to great insights on how they function, this method leaves out crucial information about how bacteria act in the real world, the study stated. Taking a different approach, researchers examined bacteria growing on toddlers’ teeth and imaged the bacteria that cause tooth decay-the biofilm known as dental plaque- in 3D in their natural environment and how it formed on toddlers’ teeth that were affected by caries.
“We started with these clinical samples, extracted teeth from children with severe tooth decay,” said Hyun (Michel) Koo, D.D.S, MS, Ph.D., a professor in the Department of Orthodontics at UPenn School of Dental Medicine and a co-senior author of the study. “The question that popped in our minds was, ‘How these bacteria are organized and whether their specific architecture can tell us about the disease they cause?’”
The team used a combination of super-resolution confocal and scanning electron microscopy with computational analysis to dissect the arrangement of S. mutans and other microbes of the intact biofilm on the teeth. This approach allowed the team to study the biofilm layer by layer while building a 3D picture of the architectures.
During the study, researchers found that S. mutans in dental plaque most frequently appeared in a mound against the tooth’s surface, but not on its own. While S. mutans formed the inner core of the rotund architecture, other bacteria, such as S. oralis formed outer layers arranged in a crown-like structure. Supporting and separating these layers was an extracellular scaffold made of sugars produced by S. mutans, effectively encasing and protecting the disease-causing bacteria.
“We found this highly ordered community with a dense accumulation of S. mutans in the middle surrounded by these ‘halos’ of different bacteria, and wondered how this could cause tooth decay,” Dr. Koo said.
The research team attempted to recreate the natural plaque formations on a tooth-like surface in the lab setting using S. mutans, S. oralis, and a sugar solution to learn more about how structure impacted the function of the biofilm. They successfully grew the formations, with rotund-shaped architecture and crown-like structure, and then measured levels of acid and demineralization associated with them.
“What we discovered, and what was exciting for us, is that the rotund areas perfectly matched with the demineralized and high acid levels on the enamel surface,” Dr. Koo said. “This mirrors what clinicians see when they find dental caries: Punctuated areas of decalcification known as ‘white spots.’ The crown-like structure could explain how cavities get their start.”
In the final experiment, researchers put the community to the test by applying an antimicrobial treatment and observing how the bacteria fared. When the crown-like structures were intact, S. mutans in the inner core largely avoided dying from the antimicrobial treatment. Only by breaking down the scaffolding material holding the outer layers together, were they able to ensure the antimicrobial treatment permeated and killed the cavity-causing bacteria.
The study could help researchers more effectively target the pathogenic core of dental biofilms, but it may also have implications for other fields, according to Dr. Koo.
“It demonstrates that the spatial structure of the microbiome may mediate function and the disease outcome, which could be applicable to other medical fields dealing with polymicrobial infections,” said Dr. Koo.
The study can be read in its entirety here. For more information, visit the UPenn School of Dental Medicine at dental.upenn.edu.