RevBio Receives FDA Approval for Clinical Trial on Tetranite Implant Stabilizing Biomaterial
This biomaterial will undergo testing to see if it will be suitable for use in immediate loading implant situations for patients.
RevBio Receives FDA Approval for Clinical Trial on Tetranite Implant Stabilizing Biomaterial. Image: © RevBio, Inc.
RevBio has received approval from the United States Food and Drug Administration (FDA) to begin clinical trials of its bone adhesive biomaterial Tetranite®. Tetranite, which is described as “osteopromotive” in a press release from RevBio, will be tested by industry veterans Kanyon Keeney, DDS, and Paul Fugazzotto, DDS for the FDA. “The adhesive properties and handling characteristics of this material are incomparable to any product on the market,” Dr Fugazzotto says in the press release.
With Tetranite, RevBio aims to stabilize implant sites and reduce the time it takes for effective dental implant procedures. It uses a pH modified porous formulation to stabilize unstable implants and hopefully enable immediate implant placement. This clinical trial, comprised of 20 patients, will put Tetranite to the test.
“We are truly excited to conduct the study with both Dr Fugazzotto, who will initiate this study enrolling the first cases, along with Dr Keeney,” Alan Pollack, RevBio’s Senior Director of Clinical Operations says in the press release. “The improvements we have made to the technology have accelerated both the bone substitution profile and the product’s adhesive strength. Having also just recently received IRB approval, we look forward to our first patients in the coming weeks.”
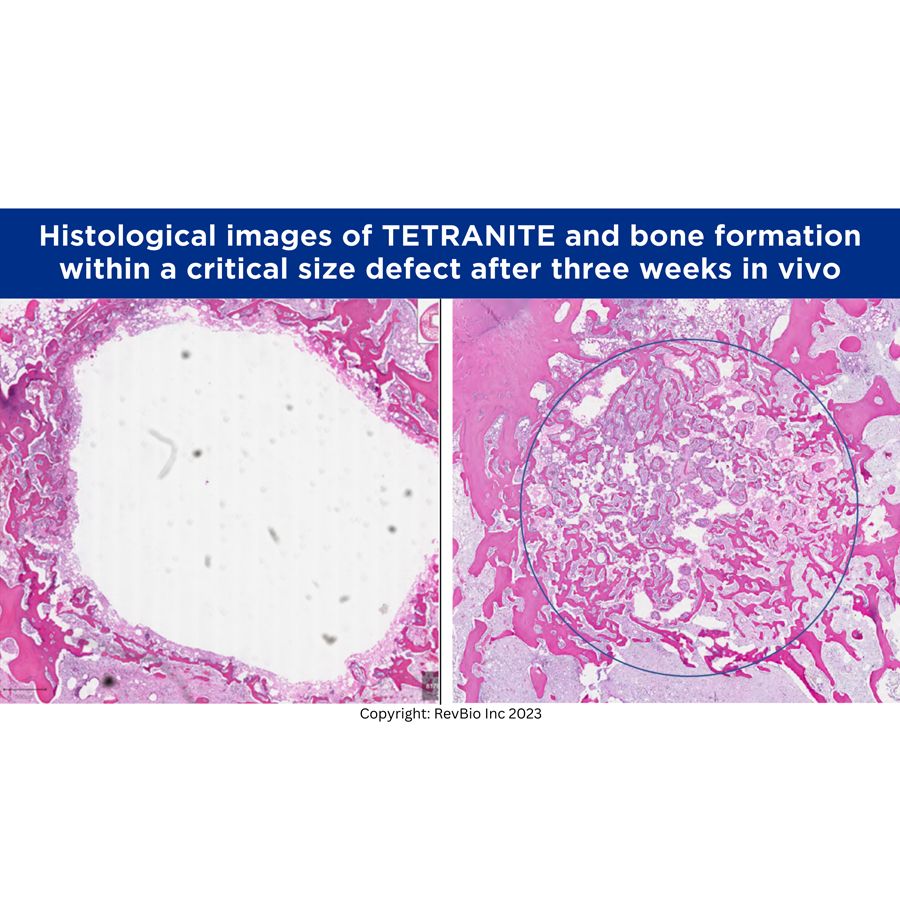
RevBio’s Tetranite is an injectable bone adhesive that has been developed for use in a variety of medical applications, including dental, orthopedic, and animal health. Early testing has shown “significant bone in-growth” per the press release.