Pearl’s Second Opinion Artificial Intelligence Software Gains FDA Approval
Pearl’s artificial intelligence system for dental condition identification has been granted FDA approval for widespread use
Pearl’s Second Opinion Artificial Intelligence Software Gains FDA Approval
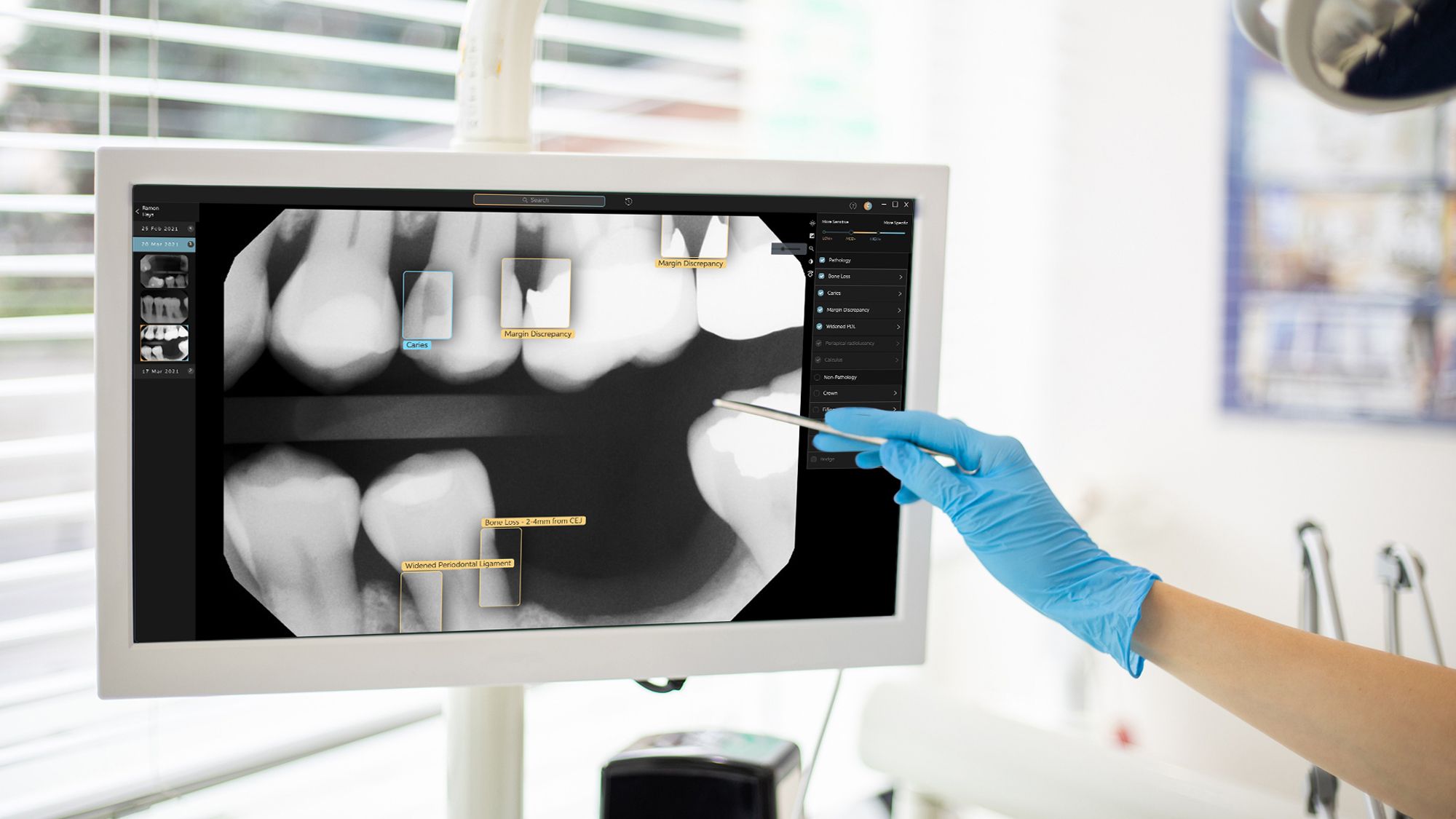
Artificial intelligence (AI) solutions company Pearl has announced that its pathology detection system Second Opinion® has been approved by the United States Food and Drug Administration (FDA). Second Opinion uses its AI to identify common dental conditions in patient x-rays. Second Opinion will demonstrate the utility of AI in the medical and dental space, according to CEO and founder of Pearl Ophir Tanz.
“This clearance is a major milestone not only for our team and for the many dentists, advisors and partners who have contributed to Second Opinion’s development, but also for dentistry itself,” Tanz said in a press release from the company. “AI is a paradigm-shifting technology that will add value across the entire healthcare continuum. Because x-rays are a regular part of every dental patient’s experience, the first place most people will encounter the power of medical AI technology will be in their dentist’s chair. Second Opinion’s FDA clearance has made that possible.”
Second Opinion is designed to identify and highlight dental caries, margin discrepancies, calculus, periapical radiolucency, crowns, fillings, root canals, bridges, and implants. This will allow dental professionals to gain a better image of their patient’s mouth and to make treatment planning more efficient.
The FDA evaluated Second Opinion across 4 clinical studies, and is available in the U.S. now. The technology, which exists in the medical space, can now be efficiently applied to the dental space, according to Pearl’s Chief Technology Officer and co-founder Cambron Carter.
“Second Opinion now joins a family of FDA cleared CADe medical systems already in use for radiologically-driven tasks such as lung nodule detection and mammography interpretation,” Carter said in the press release. “State of the art algorithms that currently assist in the detection of cancerous lesions can now be applied to detect many more frequently occurring dental disease. The standard of care in dentistry is about to level up.”