Overjet Unveils FDA Approval for Innovative PARL Segmentation Capability
Dental artificial intelligence company’s PARL Assist is designed to detect and outline instances of PARL on periapical radiographs for patients ages 12 and above.
Overjet Unveils FDA Approval for Innovative PARL Segmentation Capability | Image Credit: © Overjet
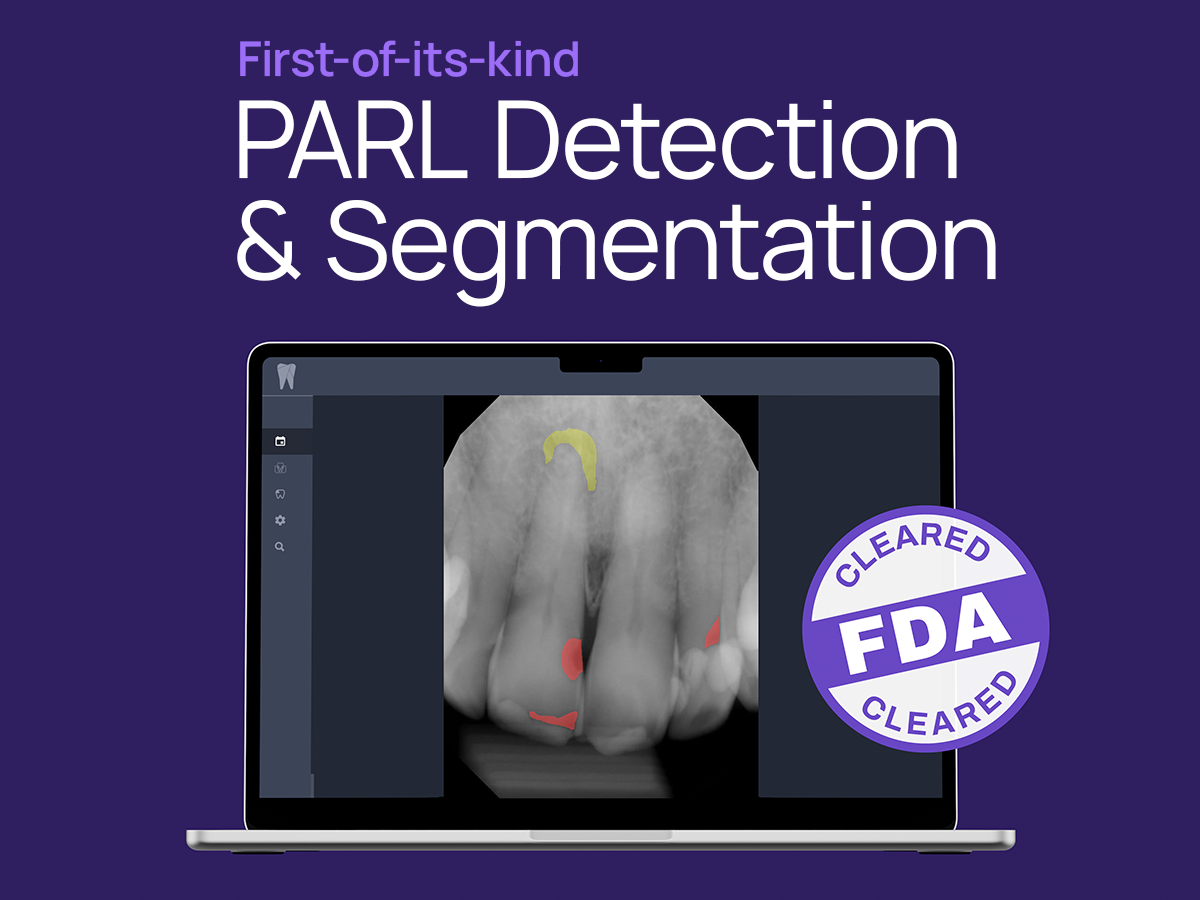
Dental artificial intelligence (AI) frontrunner Overjet has announced FDA 501(k) clearance for its groundbreaking Periapical Radiolucency (PARL) Assist device. PARL Assist is designed to aid dentists by identifying and segmenting instances of PARL on periapical radiographs. This milestone marks the company's fifth FDA Clearance, setting a record in the Dental AI space, according to Overjet.
With this recent clearance, Overjet secures its position as the first and only Dental AI provider capable of detecting and outlining instances of PARL on periapical radiographs for patients ages 12 and above. This achievement follows Overjet's trend of innovation, having become the inaugural FDA-cleared provider of AI-powered Bone Level measurements in 2021, and the sole FDA-cleared provider of AI-powered Caries Segmentation in 2022.
In the development of PARL Assist, Overjet harnessed substantial clinical expertise, rigorous testing, and validation. Expert clinicians annotated tens of thousands of clinically validated instances of periapical radiolucencies across a diverse range of clinical scenarios to train Overjet's algorithms. The reference standard testing set, establishing the quality bar for its AI, was meticulously created with multi-consensus agreement by expert US licensed endodontists, the company states.
“Dentists, for the first time in their clinical lifetimes, will be able to leverage AI in order to identify teeth that require endodontic testing and observation or potentially treatment, and utilize the precise segmentation (outlines) of the lesion to drastically improve patient communication and understanding,” Teresa Dolan, DDS, chief dental officer at Overjet, says in a press release. “Periapical lesions can be caused by tooth decay, trauma to the tooth, or a previous dental procedure, and are often ‘silent’ with patients presenting without symptoms, while potentially active infection is persisting. Beyond the clear benefits of accuracy and consistency, Overjet’s technology plays a critical role in helping doctors educate patients about their oral health,” Dr Dolan adds in the press release.
Overjet's PARL Assist product will identify and alert clinicians in various clinical scenarios, building on pre-existing clearances. Two illustrative scenarios include explicitly calling out teeth with carious lesions extending into the pulp alongside a PARL lesion and flagging teeth with pre-existing root canal therapy that potentially has unresolved PARL, prompting the clinician to investigate and test clinically.
The efficiency of the AI model and testing led to the most expeditious clearance in the dental AI space to date, spearheaded by Dr. Deepthi Paknikar, leveraging her dual expertise as an AI regulatory expert and a US licensed dentist. Dr Paknikar remarks in the press release, "We're honored to see the successful and speedy FDA clearance for PARL Assist. More innovation is on its way from Overjet, and I'm excited to support our development efforts and put new capabilities through appropriate regulatory scrutiny."
Wardah Inam, PhD, founder and CEO of Overjet, underscores the company's commitment to delivering optimal patient outcomes, saying this in the press release: "From day one, our focus has been on delivering the best outcomes for patients. That's why we continue to invest in building AI that gives dentists the best possible insights and packages these learnings in a format that patients can understand. Today, we're proud to share the world's first PARL Segmentation AI and announce its FDA clearance."
Aligned with its past major FDA-cleared solutions, Overjet collaborated closely with practicing dentists and its in-house clinical team of over 10 clinicians during the development of PARL Assist. Several Overjet customers participated in a BETA program, providing valuable feedback ahead of the official announcement.
Dentists in the BETA program noted the technology's impactful nature, especially given the challenges of detecting and diagnosing instances of PARL compared to caries. Regarding the accuracy of Overjet's detection and segmentation, a BETA dentist shared in the press release, "It was really spot on, even caught instances of PARL which I did not even pay attention to."
Overjet's new PARL Assist product is already in active use with leading dental service organizations (DSOs) and dental practices. In the coming weeks, Overjet plans to release the functionality for all its customers as a standard component in its Clinical Intelligence Platform.