Kerr Endodontics to announce two new products at CDA 2019
Traverse is said to simplify the process of establishing the glide path in root canal treatment, while the elements e-motion endodontic motor reportedly enables higher cutting efficiency.

More than 125 years ago, Kerr revolutionized endodontics with the introduction of the K-File stainless steel handfile. Today, KaVo Kerr is proud to introduce the latest innovation in endodontics with Traverse: a rotary glide path file.

Traverse is said to simplify the process of establishing the glide path in root canal treatment. Its advanced technology reportedly helps enhance the procedural technique by creating a more tapered glide path that reduces the workload of the shaping files. In addition, Traverse is engineered to reduce procedure time and manual labor when compared to traditional hand filing, resulting in improved efficiency.
Traverse is a nickel titanium file and is engineered with a triangular cross-section for better cutting efficiency, and Variable Heat Treatment technology for greater flexibility and strength. It also provides a maximum flute diameter of 1 mm and a noncutting tip to reduce the risk of ledging or perforating the canal. Traverse files are presterilized and offered in individual blister packs, with four files per pack. The Traverse system features a .25/.08 17 mm orifice opener and glide path files of .13/.06 and .18/.06 in lengths of 21 mm, 25 mm and 31 mm. With thoughtful features and design, the new Traverse rotary glide path file offers innovation, efficiency and improved Glide Path creation.
Trending article: Break out of your restorative rut
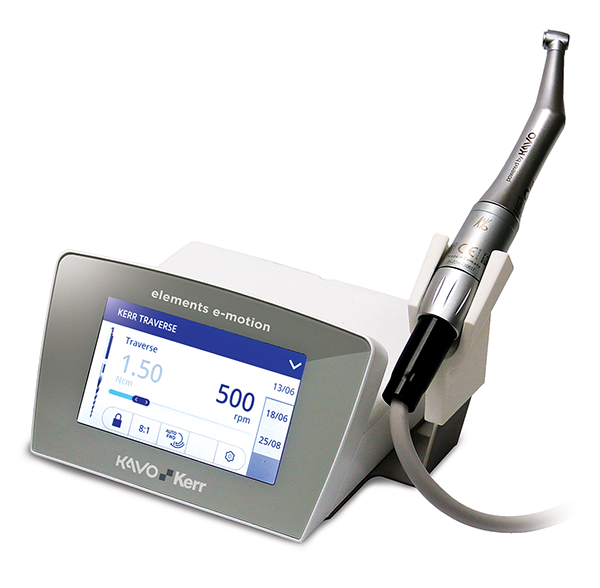
KaVo Kerr is also excited to introduce the elements™ e-motion endodontic motor with next-generation Adaptive Motion technology. The new Adaptive Motion reportedly enables higher cutting efficiency and greater protection against file separation, deformation and transportation. element e-motion also features an intuitive, full-color touch screen and a customizable interface that allows users to program up to five pre-sets with ten files each for streamlined efficiency. elements e-motion also provides the clinician with a database of 196 pre-loaded file settings, allowing maximum file sequence customizability.
Powered by KaVo’s 100 percent German-made technology, elements e-motion is built to be lighter, more compact and ergonomic when compared to other devices. It accommodates both right- and left-handed users, with four handpiece mounting options for ergonomic comfort. The smaller handpiece head also offers improved visibility and easier posterior access. With thoughtful features, Adaptive Motion technology and a remarkably intuitive interface, the new elements e-motion motor is reportedly equipped to modernize and simplify the endodontic experience.