FDA Clearance Allows Overjet Dental Assist to Bring AI to Dental Practices
The software as a medical device product applies AI in real-time to aid dentists and hygienists in diagnosis and treatment planning.
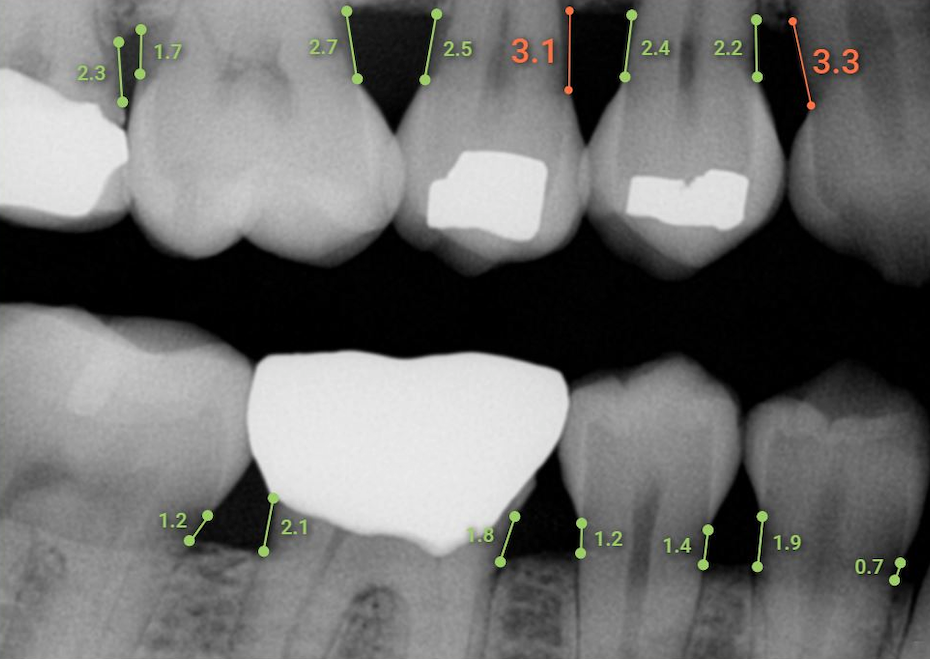
Overjet has received FDA 510(k) clearance for its Overjet Dental AssistTM artificial intelligence (AI)product. This Software as a Medical Device product applies AI in real-time to aid dentists and hygienists.
The clearance by the FDA will enable Overjet to market and sell the AI product directly to dental practices. The software supports dental professionals in measuring mesial and distal bone levels in bitewing and periapical radiographs for the diagnosis and treatment planning of periodontal disease. The condition affects an estimated 46 percent of U.S. adults, including 8.9 percent who have severe periodontitis, according to the manufacturer. Left untreated, periodontitis can lead to tooth loss and painful chewing.
“Overjet’s Dental Assist clearance by the FDA is a landmark moment for dental AI,” says Dr. Wardah Inam, PhD, CEO and co-founder of Overjet. “All clinicians can now have at their fingertips highly accurate software to detect and measure serious dental disease and clear AI visualizations to communicate with patients. This is big for dentistry and moving toward evidence-based, patient-centric care.”
In clinical performance testing, Overjet Dental Assist demonstrated automated measurement capabilities comparable to a team of highly skilled dentists. Overjet had 3 experienced dentists independently measure bone levels on dental radiographs using a measuring tool. These measurements were then further adjudicated by an oral radiologist to establish an official consensus ground truth. Overjet’s AI-powered Dental Assist measurements were then compared against this dentist consensus and had an average difference of only 0.3mm.
“We’re seeing dental AI software perform at the level of a team of trained dentists with accuracy closer than the width of a needle,” says Chris Balaban, DMD, Clinical Director for Overjet. “These tools unlock the ability to track the progression of disease over time for each tooth and make the case for evidence-based treatment, supported by unbiased software and clear visuals for patients.”
Three-year-old Overjet was founded by a team of PhDs and dentists from MIT and Harvard. They built the company around the mission of improving oral healthcare for all. To date, the company has raised over $10.5 million in venture funding and built AI models to detect a variety of dental conditions such as cavities, gum disease, tartar and impacted teeth. Overjet’s software is already in daily use by multiple large dental insurance companies to automate and improve the accuracy of dental claims review. The company has numerous partnerships with research institutions, dental support organizations and large dental practices.