Epica announces FDA clearance for SeeFactorCT3 platform
Epica International, Inc, announced its imaging platform, SeeFactorCT3™, has been given 510(k) clearance from the Food and Drug Administration (FDA).
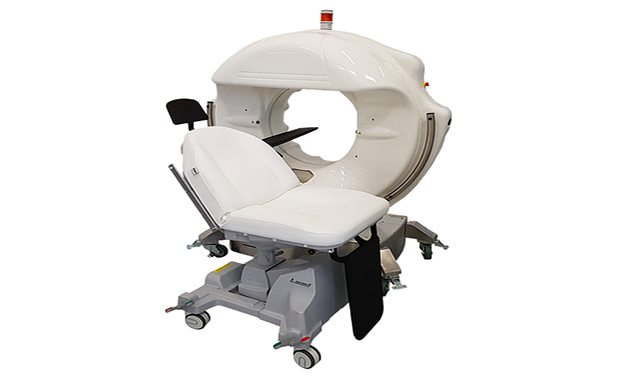
Epica International, Inc, announced its imaging platform, SeeFactorCT3™, has been given 510(k) clearance from the Food and Drug Administration (FDA), the company said in a statement.
The unit is designed to include three integrated imaging systems: CT, Fluoroscopy, and Digital Radiography. It also is said to have a detachable patient table/chair and a sterile drape for interventional procedures.
The SeeFactorCT3 is a diagnostic, interventional, and intraoperative imaging platform that reportedly secures high-resolution 3D volumetric images, plus full-featured Fluoroscopy and Digital Radiography.
The SeeFactorCT3 reportedly provides non-interpolated (slice-less, 100 percent real) image data, said to deliver isotropic image resolution as fine as 0.1mm in soft and hard tissue. Lesion detection is said to be as small as 0.2mm and Epica’s “Pulsed Technology” significantly reduces the radiation dose received.
"I am very pleased to announce this major milestone event in Epica's history; our progression into the human medical space," said Epica International CEO Frank D. D’Amelio in the press release. “What started five years ago as a multidisciplinary team of scientists, medical device engineers, robotics experts, and clinicians focused on improvement of CT imaging, led to the development of a robotically controlled, non-interpolated diagnostic and intraoperative imaging platform, capable of identifying anatomies as small as the vasculature of a hummingbird with less radiation dose and cost.”
The SeeFactorCT3 Computed Tomography platform reportedly uses dynamic flat panel sensor technology which obtains sequences of the head, dento-maxillofacial complex, teeth, mandible, jaw, and temporo-mandibular joint for use in diagnostic and intraoperative support. 2D and 3D images are displayed for each anatomical region examined.
The system is designed to be lightweight and easily maneuvered throughout a practice.
Epica International designs, develops, and distributes advanced medical imaging platforms and robotic systems that guide and assist users, ensuring accuracy and boosting outcomes. The company currently has 75 issued and pending patents on medical imaging and robotics platforms in the US and EU, the statement said.