Envista Receives FDA Clearance for Assisted Intelligence Mandibular Nerve Tracing for DTX Studio Clinic
Envista has received FDA clearance for the use of its assisted intelligence mandibular nerve tracing for its diagnostic software platform DTX Studio Clinic.
Envista Receives FDA Clearance for Assisted Intelligence Mandibular Nerve Tracing for DTX Studio Clinic
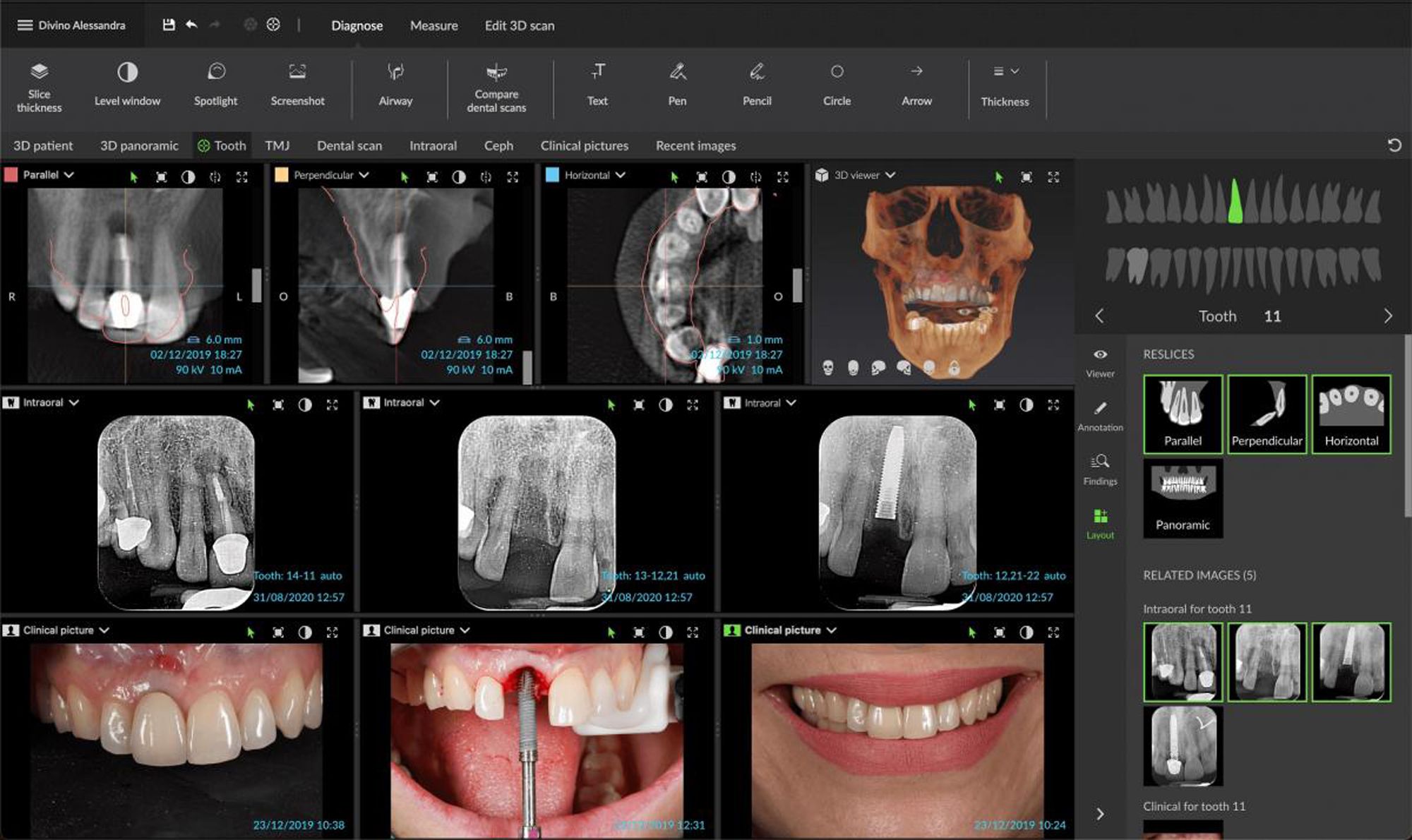
Envista Holdings Corporation has announced that it has received 510(k) Food and Drug Administration (FDA) clearance for its assisted intelligence (AI) mandibular nerve tracing feature in its software DTX Studio Clinic. DTX Studio Clinic has a variety of AI-powered functionalities, such as a virtual tooth setup algorithm, automatic tooth position identification, automatic sorting of full-mouth x-ray series, and finally a new mandibular nerve tracing on CBCT scans.
Mandibular nerve tracing visualization is designed to prevent complications and automating this process can cut down on time spent nerve tracing, according to a press release from Envista. Clinicians who use the DTX Studio image acquisition and diagnostic software platform can now take advantage of this AI-assisted mandibular nerve tracing, which is said to diversify therapeutic options for patients. Eliminating hassle was of great importance for Envista, according to Envista CEO Amir Aghdaei.
“This clearance is another important step towards our digital strategy of expanding the capabilities of our powerful, unified, and open dental diagnostic software platform DTX Studio Clinic,” Aghdaei said in the press release. “With its combination of a user-friendly interface and AI-driven functionality, DTX Studio Clinic reduces the time clinicians spend on time-consuming tasks, while simultaneously helping prevent complications and enabling increased focus on the patient. We are particularly proud of our fully automated mandibular nerve tracing functionality with its high clinical relevance for dental implant-based patient rehabilitations.”
Envista hones in its focus on digital dentistry further through this FDA clearance.