5 equipment considerations to improve infection control
How you handle and clean your equipment can have a big impact on managing infection control at your practice.
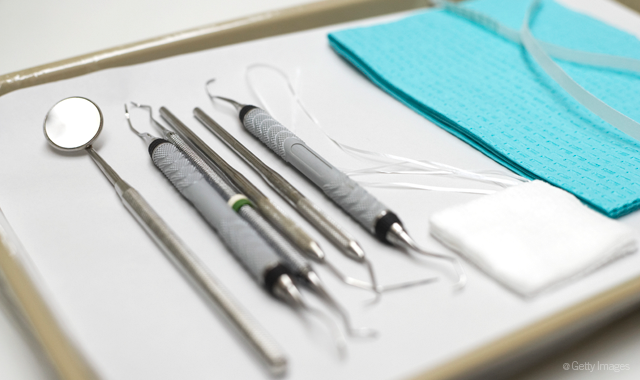
Keeping your patients safe from infection encompasses a number of considerations, including materials, procedures and equipment. What you do-or don’t do-with your equipment is an important dynamic when it comes to managing infection control.
Cassettes improve efficiency and save time
Instrument washers are certainly convenient and efficient, but just by virtue of how you use them you may be unwittingly wasting time.
Doug Braendle, product manager at SciCan, observes that being able to presort and organize instruments before going into an instrument washer-such as SciCan’s Hydrim model-eliminates a speed and efficiency bottleneck.
“The most expensive thing in the dental office is labor, it’s the staff,” Braendle says. “To put loose instruments in a Hydrim is almost a lost cause, because the advantage of a Hydrim is we put a cassette in there that has a hygiene setup. After it gets done drying, we take the whole cassette out as is, wrap it and then move on to sterilization, and then we’re ready to go for it to be used again. If it’s loose instruments, then you have this big basket and what your assistant is going to have to do is pick out individual instruments and sort them by where they need to go.
Related reading: 5 more infection control mistakes you might not realize you're making
“If that’s already in a cassette, it’s done for them,” he continues. “I don’t have somebody picking through them, losing valuable time and taking a very skilled member of the dental team because they know all the instruments.”
Select equipment based on the practice’s busyness
A number of variables go into determining which equipment to use, and an important consideration is your practice’s activity level.
“We use the term ‘pass-through,’” Braendle says. “How much stuff is passing through your system? Passing through whatever washing that you’re doing? Passing through the sterilization area and going out to be used on patients? Once we have an idea as to how busy the practice is, then it’s just a numbers game. We can help them select the proper sterilization equipment and the proper washing equipment in order to keep up with how busy they are.”
The busier a practice, the more a few minutes here and there can add up.
“We run across ortho offices that are seeing up to 150 patients a day,” Braendle says. “And when I stick an instrument, whether it be a mirror or something else, in a patient’s mouth, at some point in the protocol that involves cleaning and it involves sterilization. We have to know what the pass-through is in order for us to give them some guidelines as to what it is that they may or may not need in the way of sterilization.”
Set up your sterilization area
A smooth, logical flow to your sterilization can also save time and money.
“When you’re dealing with sterilization centers from an infection control standpoint, from the CDC and what the state mandates, it’s really imperative that we have a dirty-to-sterile protocol,” Braendle says. “It can be left to right, it can be right to left. It all depends on how the room is set up. There’s no right or wrong, it just depends on the flow of that room.”
Being aware of your sterilization area’s size and configuration is an important variable.
“Oftentimes with new setups, washers are fairly common,” Braendle says. “When you’re trying to redo an existing office, a washer can be somewhat problematic, because it’s the size of a regular dishwasher that you might have in your home. In some of these smaller sterilization areas, there may not be a space that big where we can just slide new things in.”
When the area is properly organized, the workflow should be smooth and fluid.
“Once the sterilization center is thought of, we’ll normally have a dirty storage, so the dirty cassettes come in, and if they have time to clean them and get some of the merchandise out-like two by twos and cotton rolls and things like that-rinse equipment off and then put it into the washer. We can do rinse and holds in between. When the machine is full enough, pick a cycle and then walk away,” Braendle says.
Continue to page two to read more...
Follow manufacturer’s instructions exactly
Kathy Eklund, RDH, director of occupational health and safety at The Forsyth Institute, points out that following the precise directions for cleaning equipment is critical.
“When you’re doing training, you have to be sure that everybody has access to those instructions,” Eklund says. “So for things like dental handpieces and other types of semi-critical devices that are involved and need to be sterilized, those instructions for processing are in the sterilization area. It’s not just that you’re sterilizing them. There are specific cleaning instructions, specific maintenance instructions, specific lubrication that might be needed. That all plays into ensuring that they are properly processed and are going to function most efficiently.”
Manufacturers also provide validated processing instructions that must be followed.
“These are the instructions that have been tested for effectiveness, for cleaning as well as sterilization,” Eklund says. “Those validated processing instructions allow that item to be reprocessed and reused, according to those instructions.
More from the author: 10 questions you need to ask about infection control
“If the manufacturer cannot provide validated reprocessing instructions,” she continues, “then that item-that bur, that diamond, that endo file-should be considered a single-patient use item.”
Not following manufacturer’s instructions for sterilization can lead to major problems.
“The important thing to remember is that if someone just decides on their own, ‘Well, I’ve always sterilized these, even though I don’t have instructions, I’m just going to continue to sterilize them,’ then in that case, you’d be using these items off-label,” Eklund says. “And if you would have a complication, such as something snapping or breaking in the mouth, it would get aspirated, or something around those lines, you’ve used the product off-label, which would be a tremendous risk management issue.”
Handpieces
Handpieces are the workhorses of the dental practice and, as such, are critical to clean properly.
“Any dental handpiece that is removable from an air or water line on the unit, CDC recommendations have been, for a very long time-since as early as 1986, rerated in 1993 and 2003 as well, and more recently in their summary publication in March 2016-that these items should be sterilized between each patient use according to manufacturer’s instructions,” Eklund says.
The level of traffic the practice encounters, along with the number of handpieces they own, can make sterilization challenging.
“With the era of low-speed handpieces, there has been some end-user confusion about this,” Eklund says. “They have to be sterilized between each patient, according to the manufacturer’s instructions. And with dental handpieces in particular, they are an expensive item, and if the practice does not have the appropriate inventory, or enough sterilizers to run enough sterilization cycles to keep up with the inventory that they do have, the end user may think that it’s okay just to wipe them down, and that’s not appropriate. It’s not following manufacturer’s instructions. The other component of it is understanding the concern for contamination is not just the outside, but also the inside of the handpiece where you can have retracted oral fluids or other contamination inside the handpiece itself, and that’s the reason and the rationale behind the sterilization.”
For more information about equipment safety and infection control, visit the Organization for Safety, Asepsis and Prevention (OSAP)’s website at www.osap.org.
The Centers for Disease Control (CDC) offers a number of infection control guidelines in its Summary of Infection Prevention Practices in Dental Settings, located at www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care.pdf.